分子别名(Synonym)
C-C chemokine receptor type 2,C-C CKR-2,CC-CKR-2,CC-CKR-2CKR2B,CCR2,CCR-2,CCR2A,CCR2B,CD192 antigen,CD192,chemokine (C-C motif) receptor 2,CKR2,CKR2A,CMKBR2,CMKBR2MGC111760,FLJ78302,MCP-1 receptor,MCP-1-R,MCP-1-RMGC103828,MGC168006,Monocyte chemoattractant protein 1 receptor,monocyte chemotactic protein 1 receptor
表达区间及表达系统(Source)
Human CCR2 Full Length Protein (VLP) (CC2-H52P3) is expressed from human 293 cells (HEK293). It contains AA Met 1 - Leu 360 (Accession # P41597-2).
Predicted N-terminus: Met 1
蛋白结构(Molecular Characterization)
Virus-like particles(VLPs) are formed by self-assembly of envelop/capsid proteins from viruses. Membrane Proteins can be constituted in-situ with VLPs produced from HEK293 cell cultures. These VLPs concentrate conformationally intact membrane proteins directly on the cell surface and produce soluble, high-concentration proteins perfect for immunization and antibody screening.

The VLPs provide the display of properly folded membrane proteins in their native cellular membrane in a compact size of 100~300 nm diameter (similar to the size of most viruses) making it optimal targets for dendritic cells in vivo and surface attachment for phage display.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
制剂(Formulation)
The VLPs are highly immunogenic, so the immunization strategy should be optimized (antigen dose, regimen and adjuvant).
Supplied as 0.2 μm filtered solution in PBS, pH7.4 with glycerol as protectant.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- The product MUST be stored at -70°C or lower upon receipt;
- -70°C for 12 months under sterile conditions.
质量管理控制体系(QMS)
活性(Bioactivity)-ELISA
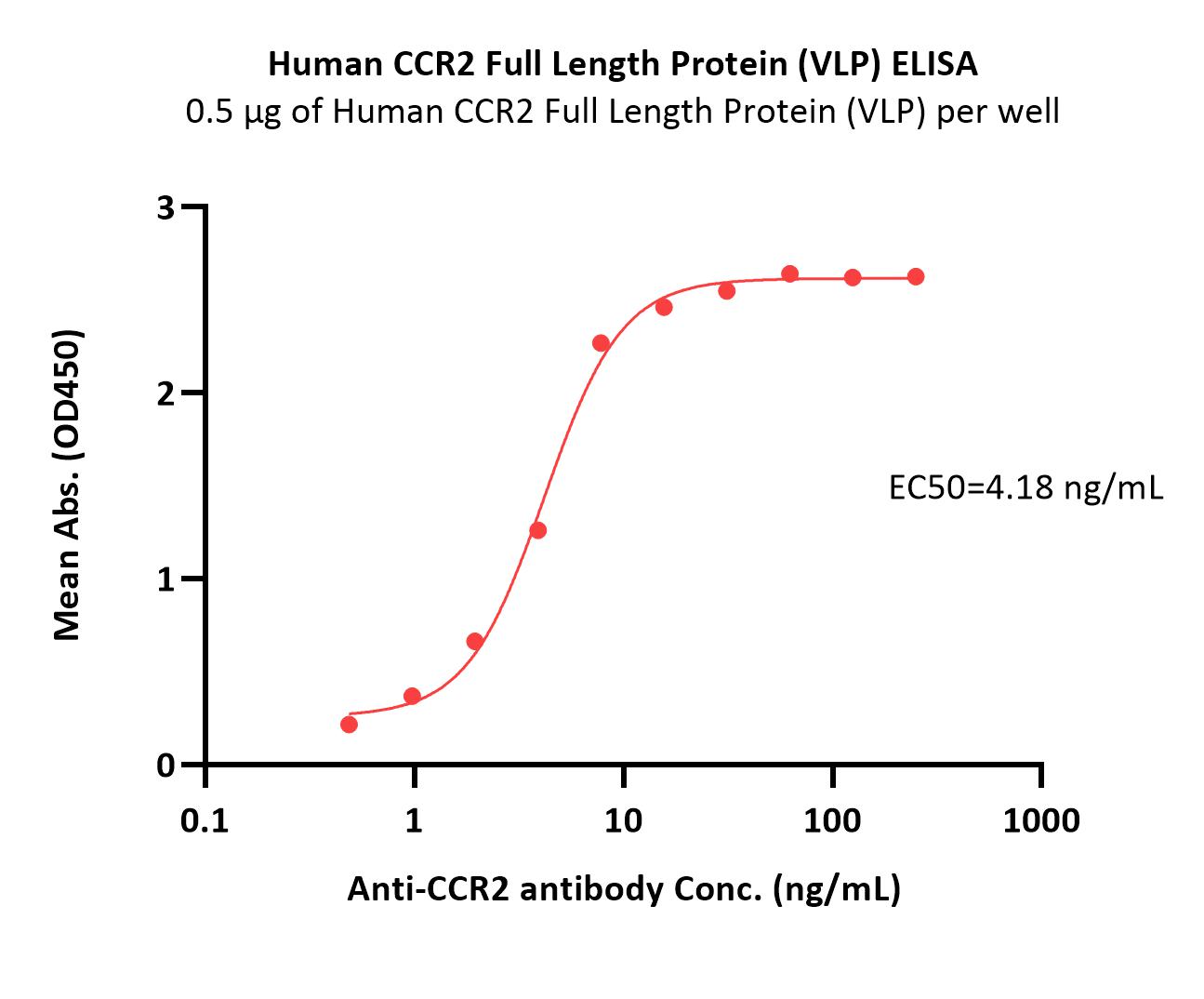
Immobilized Human CCR2 Full Length Protein (VLP) (Cat. No. CC2-H52P3) at 5 μg/mL (100 μL/well) can bind Anti-CCR2 antibody with a linear range of 0.5-8 ng/mL (QC tested).
Protocol
均一性(Identity)-DLS
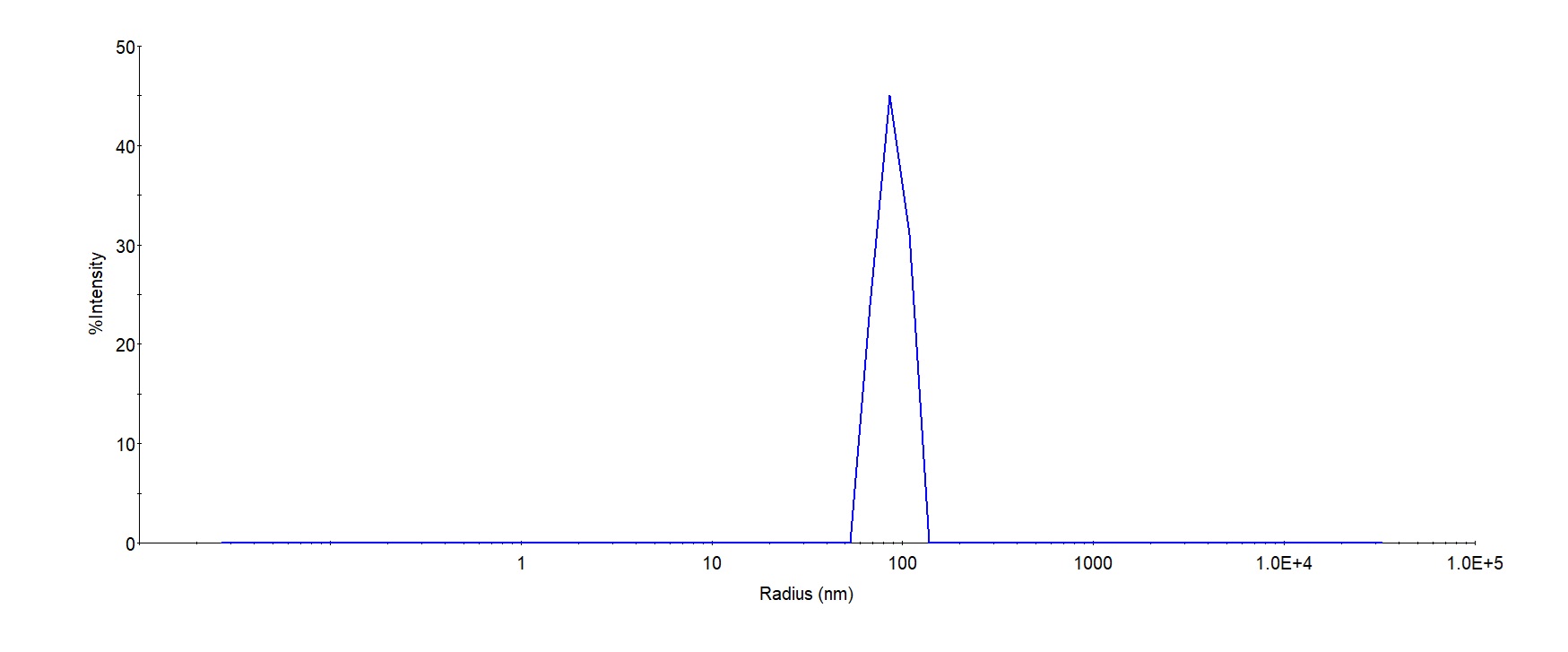
The mean peak Radius of VLP is 80-120 with more than 95% intensity as determined by dynamic light scattering (DLS).
背景(Background)
The protein encoded by this gene is a receptor for monocyte chemoattractant protein-1, a chemokine which specifically mediates monocyte chemotaxis. Monocyte chemoattractant protein-1 is involved in monocyte infiltration in inflammatory diseases such as rheumatoid arthritis as well as in the inflammatory response against tumors. The encoded protein mediates agonist-dependent calcium mobilization and inhibition of adenylyl cyclase. This protein can also be a coreceptor with CD4 for HIV-1 infection. This gene is located in the chemokine receptor gene cluster region of chromosome 3.























































 膜杰作
膜杰作 Star Staining
Star Staining















