IGF1 Signaling Regulates Neuropeptide Expression in Hypothalamic Neurons Under Physiological and Pathological ConditionsHe, Loganathan, Belsham
Endocrinology (2025) 166 (5)
Abstract: Insulin-like growth factor 1 (IGF1) plays a critical role in metabolism and aging, but its role in the brain remains unclear. This study examined whether hypothalamic neurons respond to IGF1 and how its actions are modulated. RT-qPCR and single-cell RNA sequencing indicated that Igf1r mRNA is expressed in neuropeptide Y/Agouti-related peptide (NPY/AgRP) neurons but has higher expression in pro-opiomelanocortin (POMC) neurons. IGF1 binding proteins Igfbp3 and Igfbp5 were significantly expressed, whereby Igfbp5 levels were modulated by fasting, nutrient availability, and circadian rhythms, implying that IGF1 signaling can be controlled by multiple mechanisms. In mouse and human models, IGF1 regulated Agrp, Npy, Pomc, Cartpt, Spx, Gal, and Fam237b expression, producing an overall anorexigenic profile. Hyperinsulinemia induced IGF1 resistance, accompanied by reduced IGF1R protein, as well as Igf1r and Irs2 mRNA expression via over-activation of phosphoinositide 3-kinase/forkhead box O1 (PI3K-FOXO1) signaling. Thus, hypothalamic neurons respond to IGF1 under physiological conditions, and hyperinsulinemia is a novel mechanism that drives cellular IGF1 resistance.© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Targeting insulin-like growth factor 1 receptor restricts development and severity of secondary lymphedema in miceYuan, Levy, Yeo
et aliScience (2025) 28 (3), 111948
Abstract: Secondary lymphedema is a debilitating chronic tissue swelling in a limb caused by inadequate interstitial fluid drainage due to dysfunctional lymphatic vessels. Pathological enlargement of small lymphatics contributes to lymphatic dysfunction in secondary lymphedema, but molecular mechanisms driving this remodeling are unclear. Here, using a surgical mouse model of secondary lymphedema and whole-genome microarray, we identified the transcript for insulin-like growth factor binding protein 5 (IGFBP5), a negative regulator of insulin-like growth factor (IGF) signaling, as the most profoundly down-regulated in lymphedematous tissue. Notably, IGF signaling via IGF1 receptor (IGF1R) was previously shown to promote lymphatic remodeling. We therefore targeted IGF1R in the mouse model using the small molecule IGF1R inhibitor linsitinib. Linsitinib restricted enlargement of small lymphatics and tissue swelling during lymphedema development, likely by inhibiting IGF1R-driven vascular endothelial growth factor-C (VEGF-C) synthesis by macrophages. Importantly, linsitinib profoundly reduced tissue swelling in mice with chronic lymphedema suggesting IGF signaling as a therapeutic target for this disease.© 2025 The Authors.
Schwann cells secrete IGFBP5 to facilitate the growth of keloidsWei, Shi, Wang
et alLife Sci (2025) 369, 123534
Abstract: Keloids (KD) are noncancerous fibroproliferative tumors exhibiting cancer-like traits, encompass aggressive unregulated growth, absence of natural regression, and a significantly high rate of recurrence. The precise molecular mechanisms underlying KD pathology remain poorly understood. In this study, we employed single-cell sequencing to examine the characteristics of cells in KD and normal scar (NS) tissue. We evaluated Schwann cells and their secretory protein IGFBP5 function in KD. Then, the recombinant IGFBP5 protein was employed to elucidate the regulatory roles of IGFBP5 in the proliferation, migration, invasion, angiogenesis, and cell cycle of keloids fibroblasts (KF). The rabbit ear scar model was utilized to ascertain the function of IGFBP5 in vivo. We demonstrated that in KD, the proportion of Schwann cells was 4.13 times that of NS. Besides, the IGFBP5 gene exhibited an expression level that was 8.02 times higher in KD Schwann cells compared to those in NS Schwann cells. High IGFBP5 expression was positively associated with the cell proliferation, migration, invasion, angiogenesis, and cell cycle of KF. Additionally, the p53/p21/Cyclin D1 pathway regulated cell cycle and promoted cell proliferation, which was suppressed after rIGFBP5 administration. These findings suggest that Schwann cells infiltrate in KD and secrete IGFBP5 protein to promote KD growth, and targeting IGFBP5 or Schwann cell infiltration could offer novel therapeutic strategies for KD.Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
IGFBP5 regulates fibrocartilage differentiation and cartilage injury induced by T-2 toxin via blocking IGF-1/IGF-1R signalingWang, Wang, Lei
et alRheumatology (Oxford) (2025)
Abstract: Kashin-Beck disease (KBD) is a form of osteoarthropathy that affects the skeletal and joint systems of children and adolescents. Insulin-like Growth Factor Binding Protein 5 (IGFBP5) plays an important role in bone growth and development. This study aimed to investigate the role of IGBFP5 in regulating the function and differentiation of chondrocytes in KBD.The mRNA and protein expressions of IGFBP5, IGF-1 and IGF-1R were detected by RT-qPCR and western blot assays. Commercial kits were performed to measure the mitochondrial ROS content, calcium loading and ATP synthesis in chondrocytes. MTT assay was used to detect the cell viability of chondrocytes. Co-IP and pull-down assays were conducted to verify the binding activity of IGFBP5 to IGF-1R. The rat KBD model was constructed by a low selenium diet and T-2 toxin.The expression of IGFBP5 was upregulated in KBD patient and rat tissues. Further studies showed that interfering with IGFBP5 effectively inhibited T-2-induced chondrocyte damage and mitochondrial stress. IGFBP5 depressed the interaction between IGF-1 and IGF-1R, thereby affecting the regulation of IGF-1/IGF-1R signalling in the repair of chondrocytes. In addition, the fibrous differentiation of cartilage progenitor cells (CPCs) and the activity and migration of CPCs induced by T-2 stimulation were suppressed under IGFBP5 silence treatment.IGFBP5 was upregulated during the pathological progression of KBD, and IGFBP5 competitively bound with IGF-1R to impede the interactions between IGF-1 and IGF-1R. Knockdown of IGFBP5 inhibited fibrotic differentiation and ameliorated reduction of CPC function in KBD model.© The Author(s) 2025. Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.

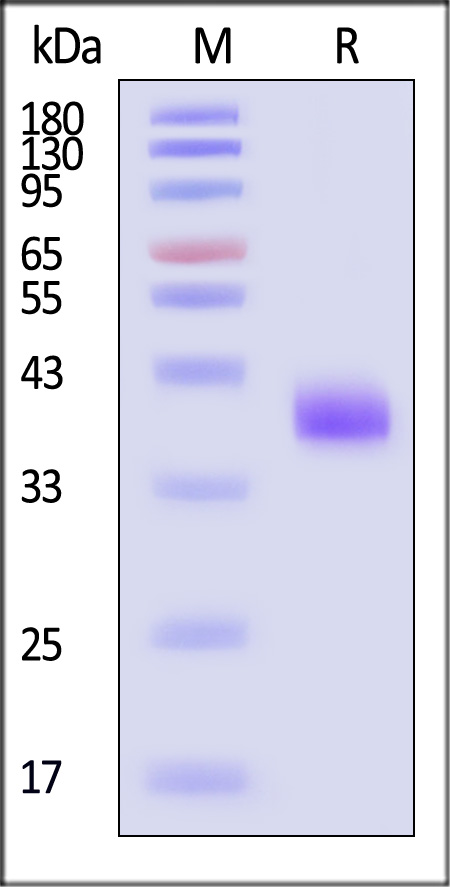
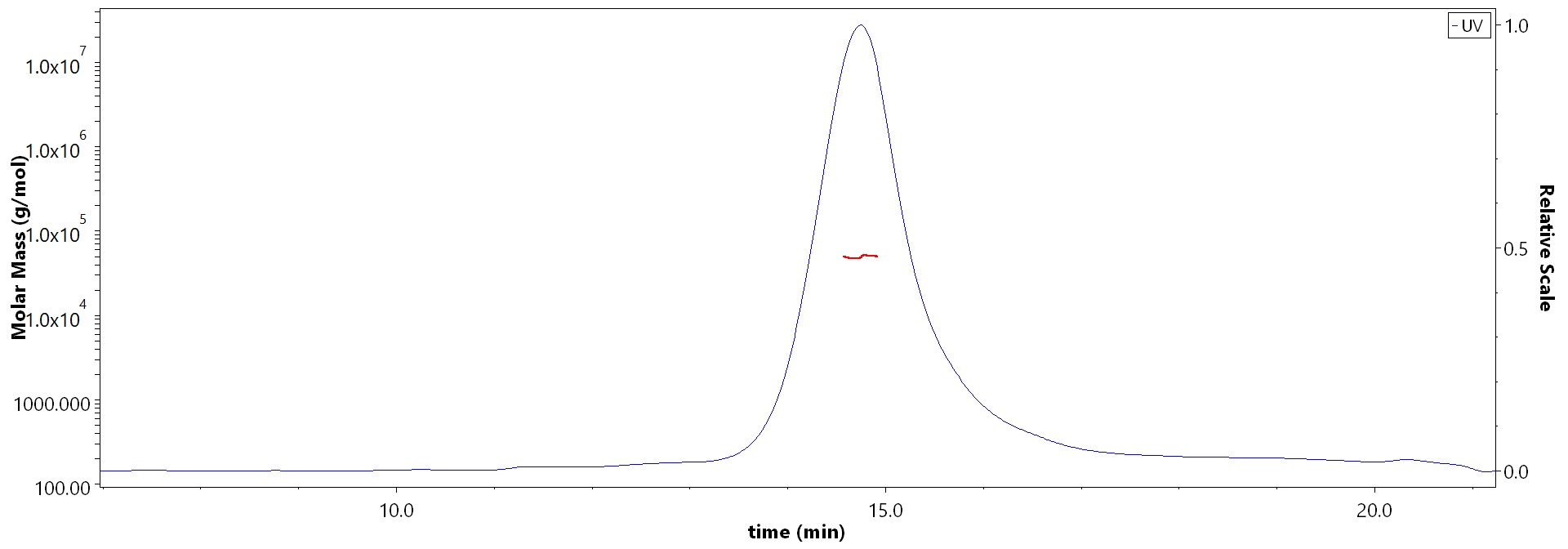
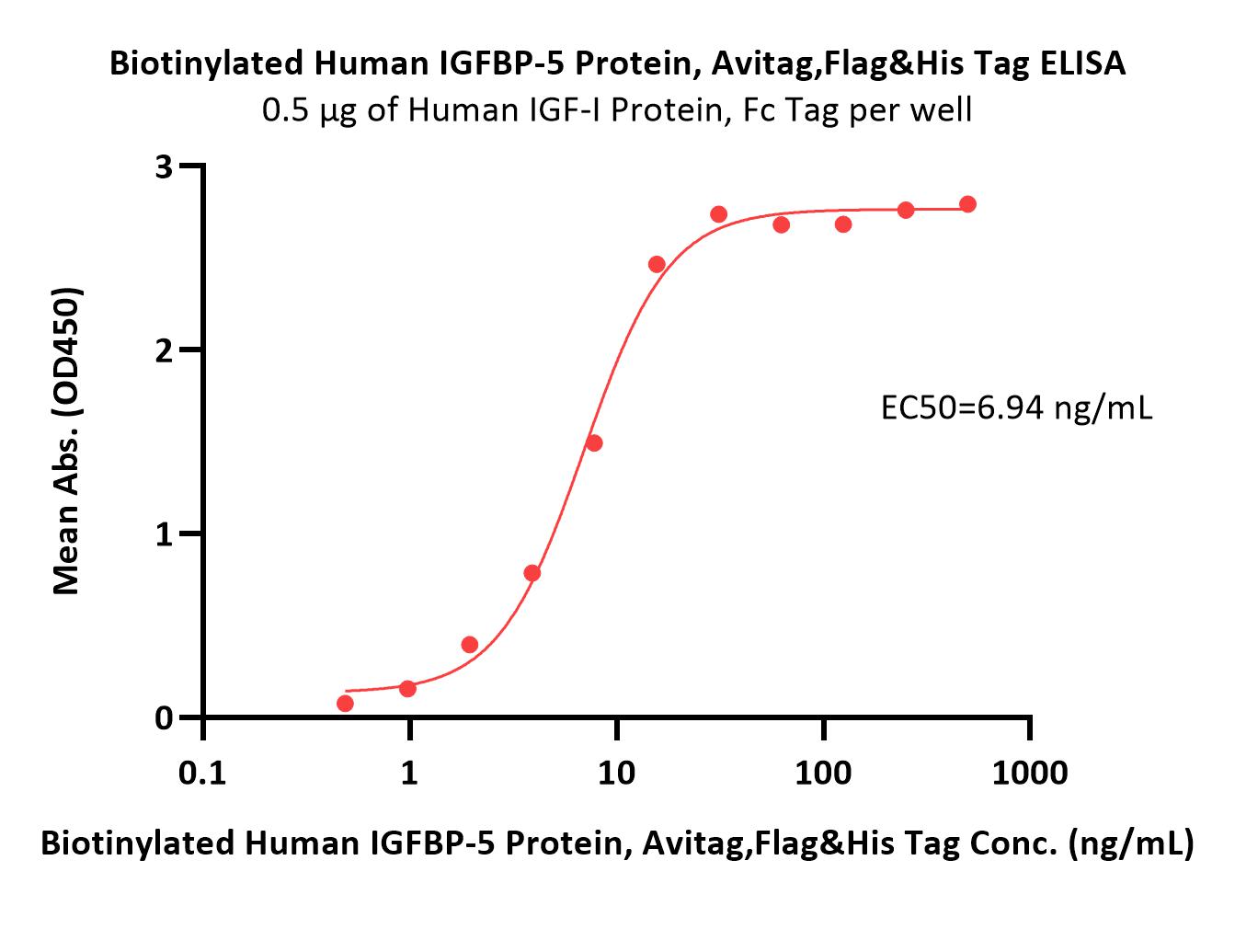























































 膜杰作
膜杰作 Star Staining
Star Staining















