- Genetically modified cell lines best reflect MOA (Mechanism of Action)
- Higher activity and larger assay window for robust and reproducible cell-based bioassay
- Comprehensive application data to support assay development and validation
- Full tracible record, stringent quality control and validated cell passage stability
- Parental cell line legally obtained from internationally recognized cell resource bank and commercially licensed
- Global commercial license assistance whenever regulatory filing is required
描述(Description)
The Human TPO R (Luc) HEK293 Reporter Cell was engineered to not only express STAT5 signaling response element, but also express the receptor full length human TPO R (Uniprot: P40238-1), which can drive luciferase expressing systems by TPO R agonists or TPO. In the absence of agonist or TPO, the TPO R receptor is not activated and luminescence signal is low. In the presence of agonist or TPO, the TPO R pathway-activated luminescence can be detected in a dose-dependent manner.
应用说明(Application)
• Screen for agonists that can bind and activate TPO R

生长特性(Growth Properties)
Adherent
筛选标记(Selection Marker)
Puromycin (2 μg/mL) + Hygromycin B (20 μg/mL)
培养基(Complete Growth Medium)
DMEM + 10% FBS
冻存液(Freeze Medium)
Serum-free cell cryopreservation medium
装量(Quantity)
1 vial contains at least 5×10^6 cells in 1 mL serum-free cryopreservation medium
存储(Storage)
Frozen in liquid nitrogen.
支原体检测(Mycoplasma Testing)
Negative
无菌检测(Sterility Testing)
Negative
使用说明(Instructions for Use)
See data sheet for detailed culturing and assay protocol.
Receptor Assay
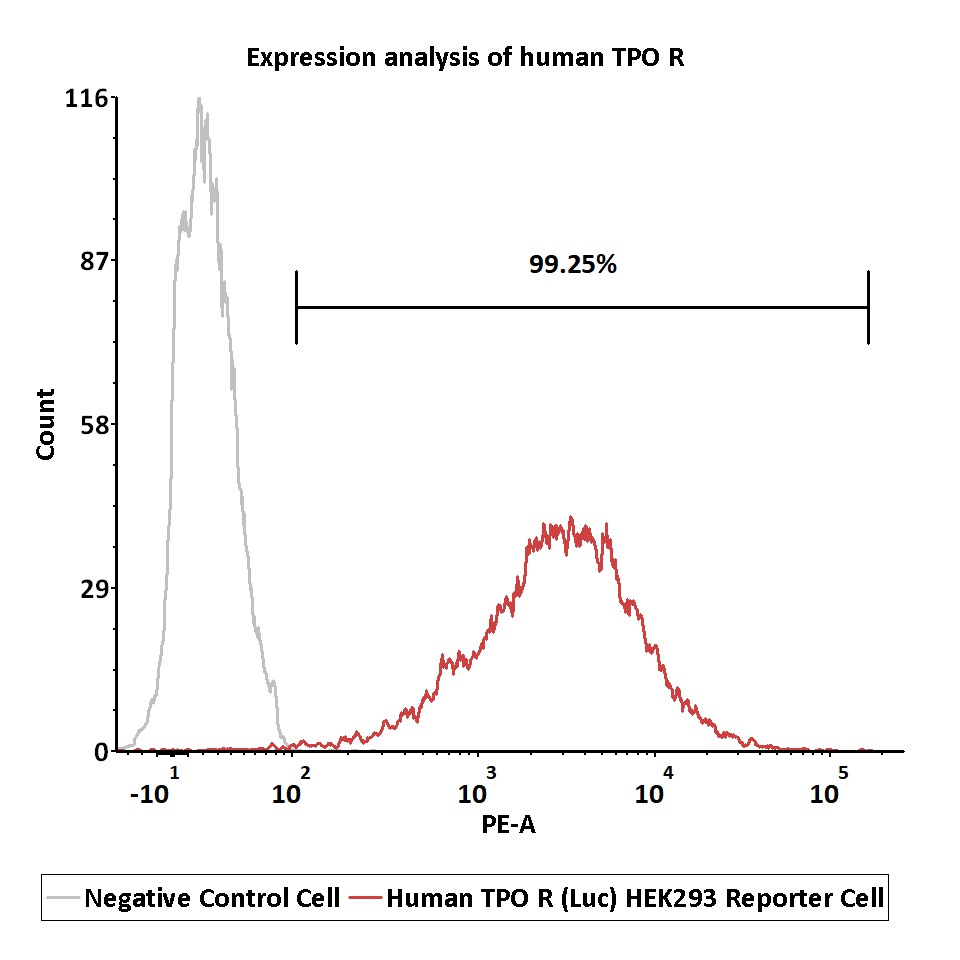
Expression analysis of human TPO R on Human TPO R (Luc) HEK293 Reporter Cell by FACS.
Cell surface staining was performed on Human TPO R (Luc) HEK293 Reporter Cell or negative control cell using PE-labeled anti-human TPO R antibody.
Protocol
Application
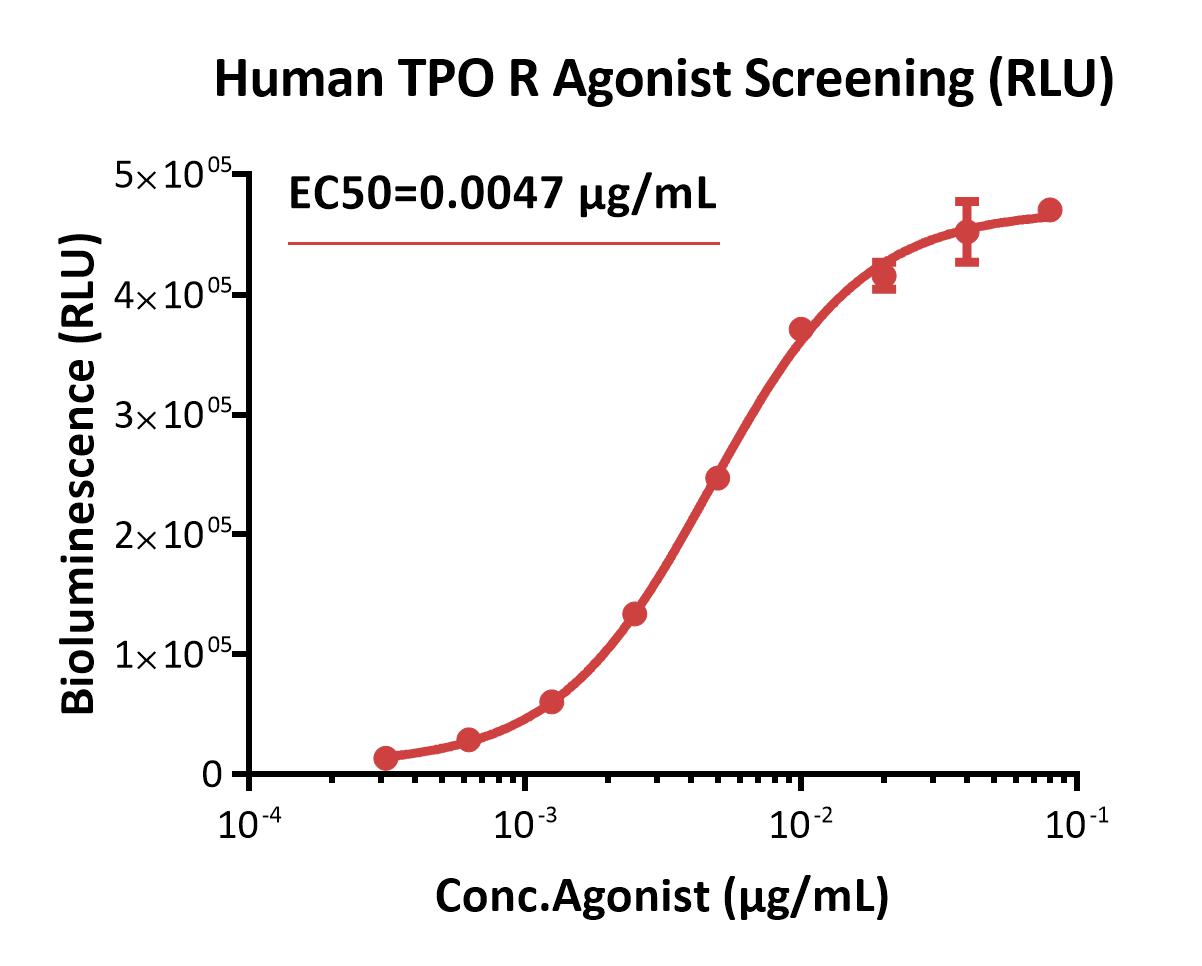
Bioactivity analysis of human TPO R agonist (RLU).
This reporter cell was incubated with serial dilutions of TPO R agonist. The EC50 of TPO R agonist was approximately 0.0047 μg/mL.
Protocol
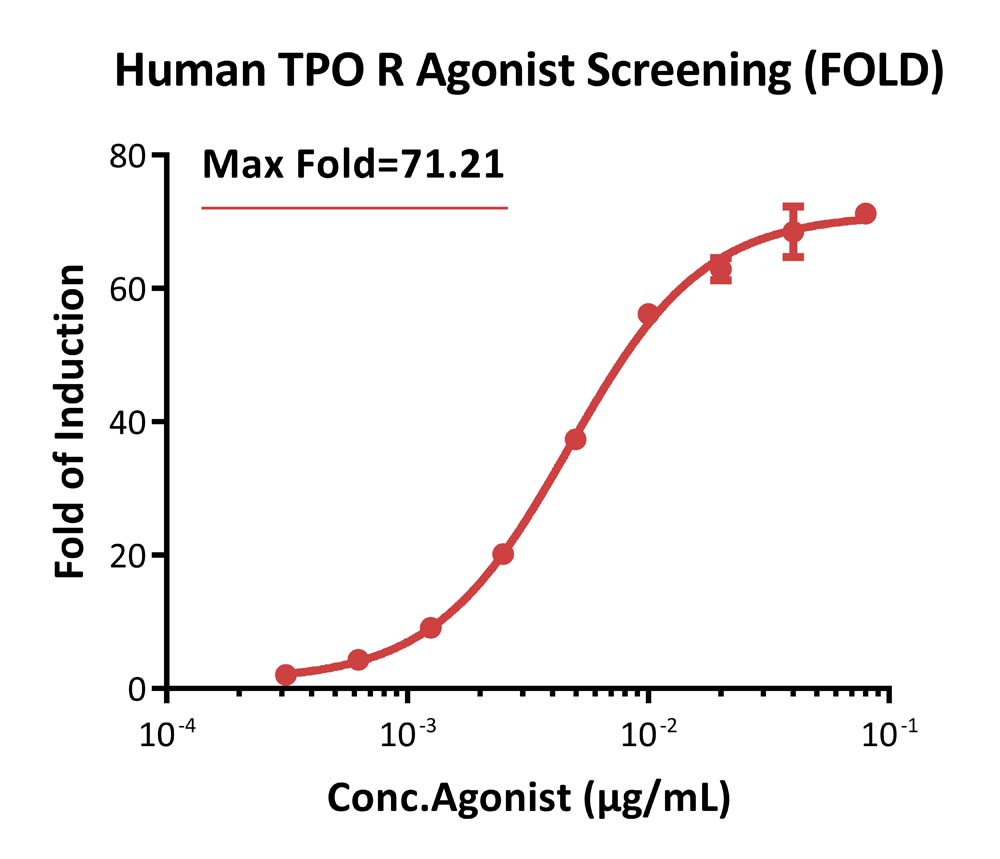
Bioactivity analysis of human TPO R agonist (FOLD).
This reporter cell was incubated with serial dilutions of TPO R agonist. The max induction fold was approximately 71.21.
Protocol
如有相关细胞池需求请联系我们
背景(Background)
Thrombopoietin R, also known as TPO-R, is expressed predominantly on the surface of MKs, platelets, hemangioblasts, and hematopoietic stem cells (HSCs). Binding of TPO to the megakaryocyte TPO-R leads to different effects: prevention of megakaryocyte apoptosis; increased megakaryocyte number, size, and ploidy; increasing rate of megakaryocyte maturation; and internalization of the TPO/TPO-R complex. Thrombopoietin R involved in multiple signal transduction pathways, such as JAK, STAT, and MAP kinase.
Limited Use&License Disclosure
BY USE OF THIS PRODUCT, RESEARCHER AGREES TO BE BOUND BY THE FOLLOWING TERMS OF LIMITED USE OF THIS CELL LINE PRODUCT.
- If the researcher is not willing to accept the terms of limited use of this cell line product, and the product is unused, ACRO will accept return of the unused product.
- Researchers may use this product for research use only, no commercial use is allowed. "Commercial use" means any and all uses of this product and derivatives by a party for profit or other consideration and may include but is not limited to use in: (1) product manufacture; and (2) to provide a service, information or data; and/or resale of the product or its derivatives, whether or not such product or derivatives are resold for use in research.
- This cell line is neither intended for any animal or human therapeutic purposes nor for any direct human in vivo use . You have no right to share, modify, transfer, distribute, sell, sublicense, or otherwise make the cell line available for use to other researchers, laboratories, research institutions, hospitals, universities, or service organizations.
- ACROBIOSYSTEMS MAKES NO WARRANTIES OR REPRESENTATIONS OF ANY KIND, EITHER EXPRESSED OR IMPLIED, WITH RESPECT TO THE SUITABILITY OF THE CELL LINE FOR ANY PARTICULAR USE.
- ACROBIOSYSTEMS ACCEPTS NO LIABILITY IN CONNECTION WITH THE HANDLING OR USE OF THE CELL LINE.
- Modifications of the cell line, transfer to a third party, or commercial use of the cell line may require a separate license and additional fees. Please contact order.cn@acrobiosystems.com for further details.























































 膜杰作
膜杰作 Star Staining
Star Staining











