分子别名(Synonym)
CTLA4,CD152
表达区间及表达系统(Source)
Biotinylated Human CTLA-4, Fc,Avitag (CT4-H82F3) is expressed from human 293 cells (HEK293). It contains AA Ala 37 - Phe 162 (Accession # P16410-1).
Predicted N-terminus: Ala 37
Request for sequence
蛋白结构(Molecular Characterization)
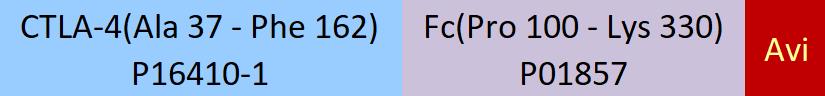
This protein carries a human IgG1 Fc tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 41.8 kDa. The protein migrates as 48-60 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in Tris with Glycine, Arginine and NaCl, pH7.5 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human CTLA-4, Fc,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
活性(Bioactivity)-ELISA
Immobilized Human B7-2, Fc Tag (Cat. No. CD6-H5257) at 5 μg/mL (100 μL/well) can bind Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) with a linear range of 0.6-10 ng/mL (QC tested).
Protocol
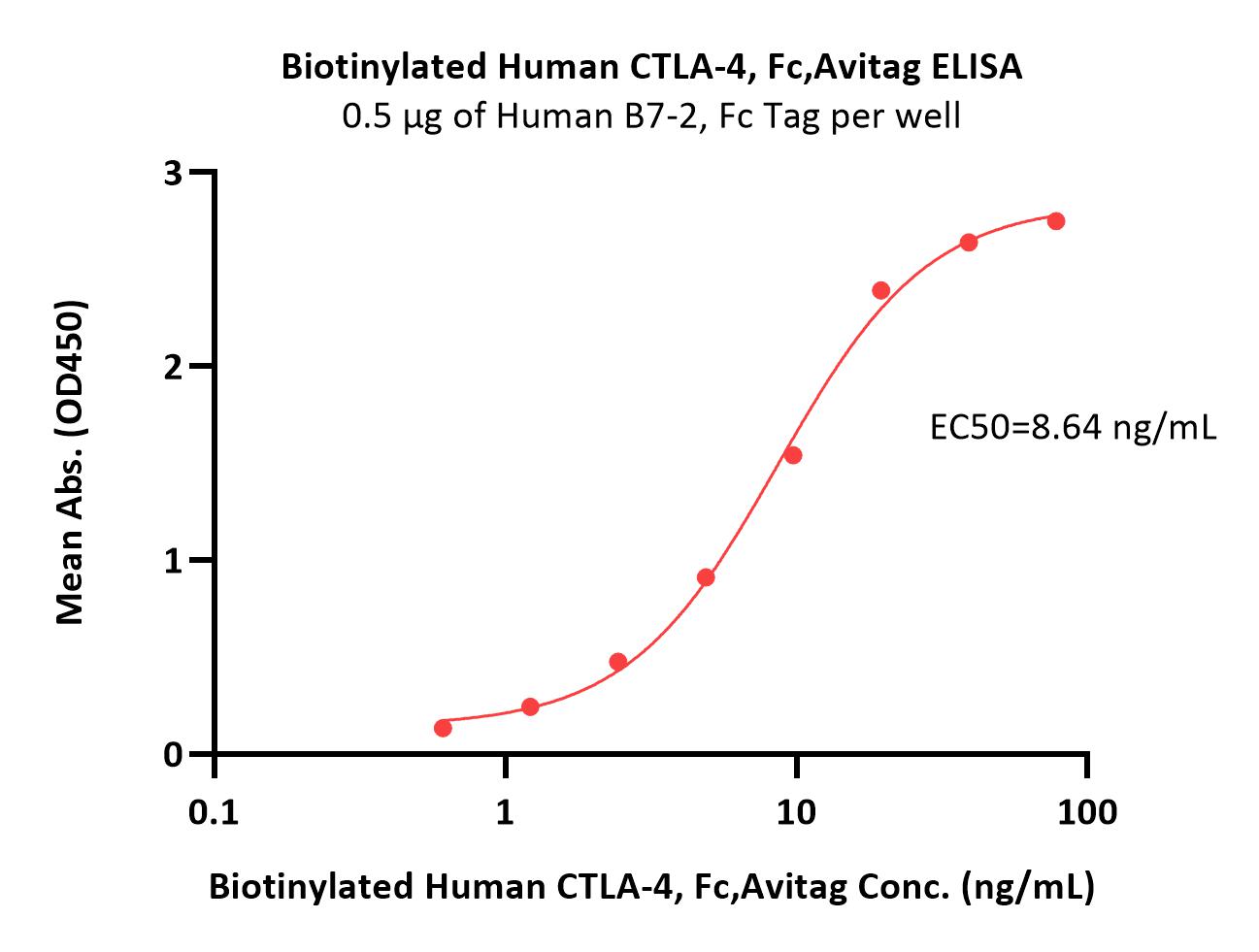
Immobilized Human B7-1, Fc Tag (Cat. No. B71-H5259) at 5 μg/mL (100 μL/well) can bind Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) with a linear range of 0.6-10 ng/mL (Routinely tested).
Protocol
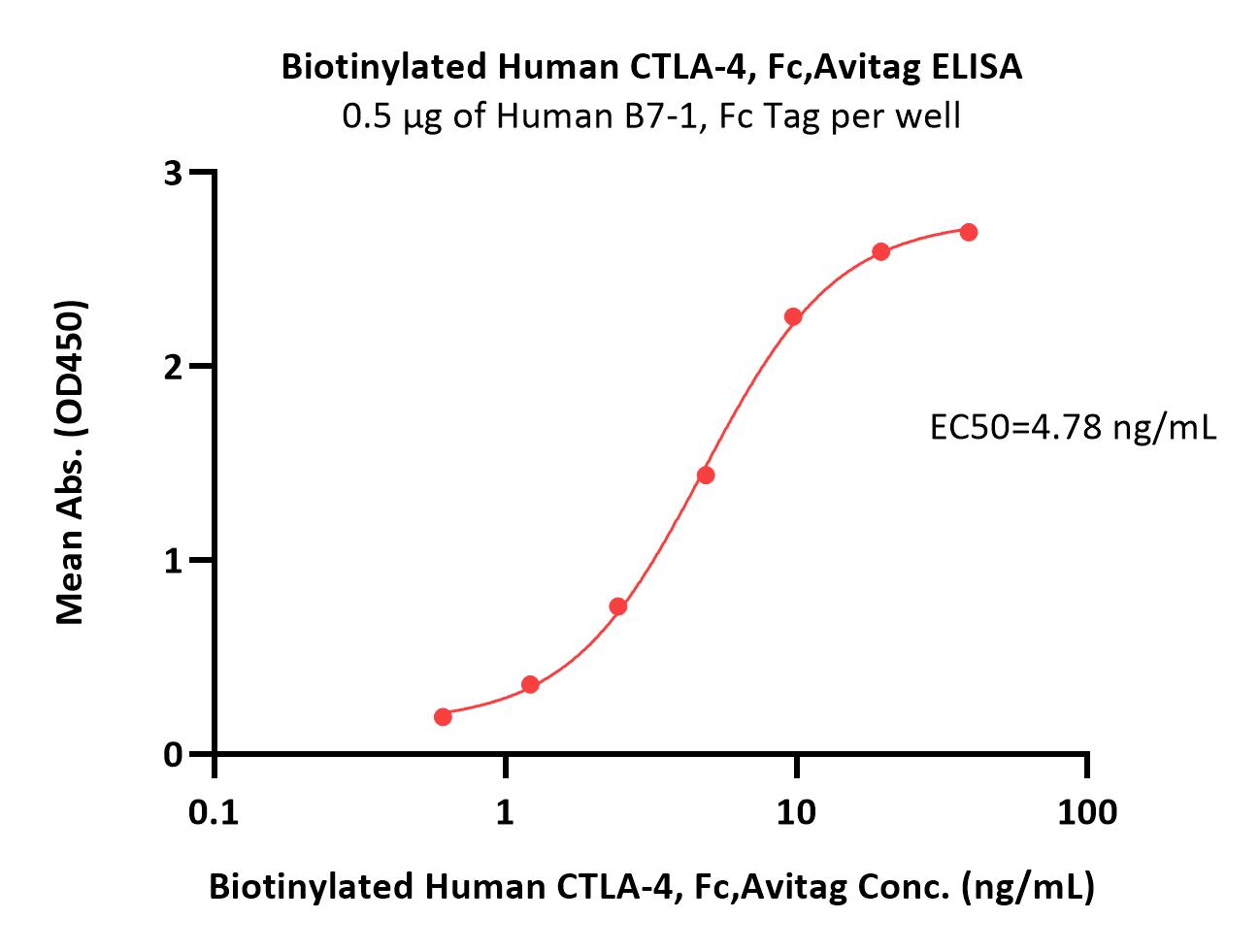
Immobilized Cynomolgus / Rhesus macaque B7-2, His Tag (Cat. No. CD6-C52H5) at 5 μg/mL (100 μL/well) can bind Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) with a linear range of 0.02-0.625 μg/mL (Routinely tested).
Protocol

Immobilized Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) at 1 μg/mL (100 μL/well) on Streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate, can bind Ipilimumab with a linear range of 0.2-2 ng/mL (Routinely tested).
Protocol
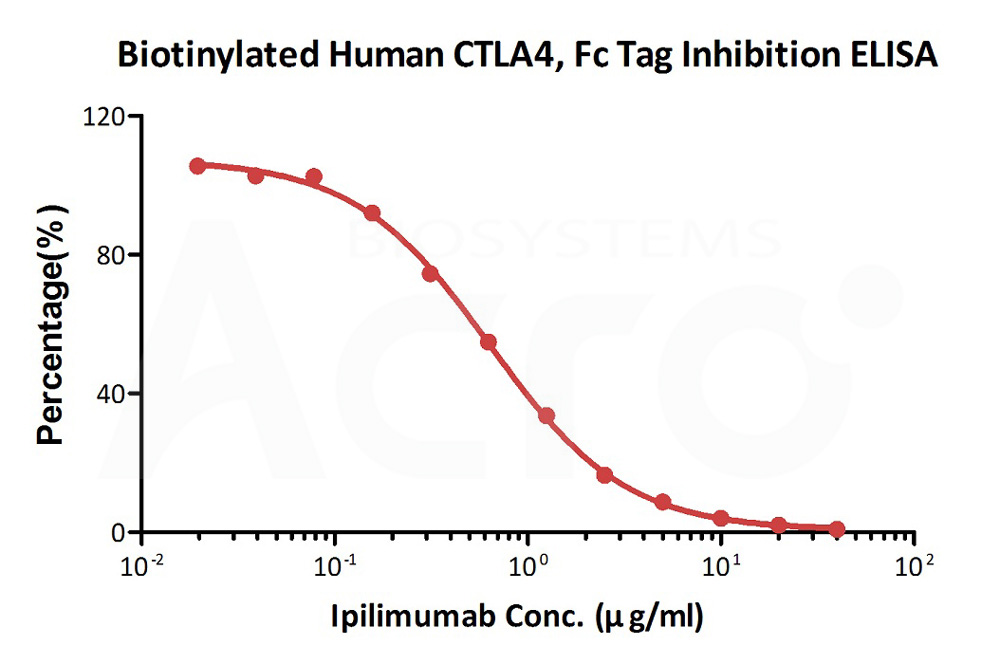
Serial dilutions of Ipilimumab neutralizing antibody (1:2 serial dilutions, from 40 μg/mL to 0.019 μg/mL) were added into Human B7-1, Fc Tag (Cat. No. B71-H5259): Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) binding reactions. The assay was performed according to the above described protocol. Background was subtracted from data points before curve fitting.
Protocol
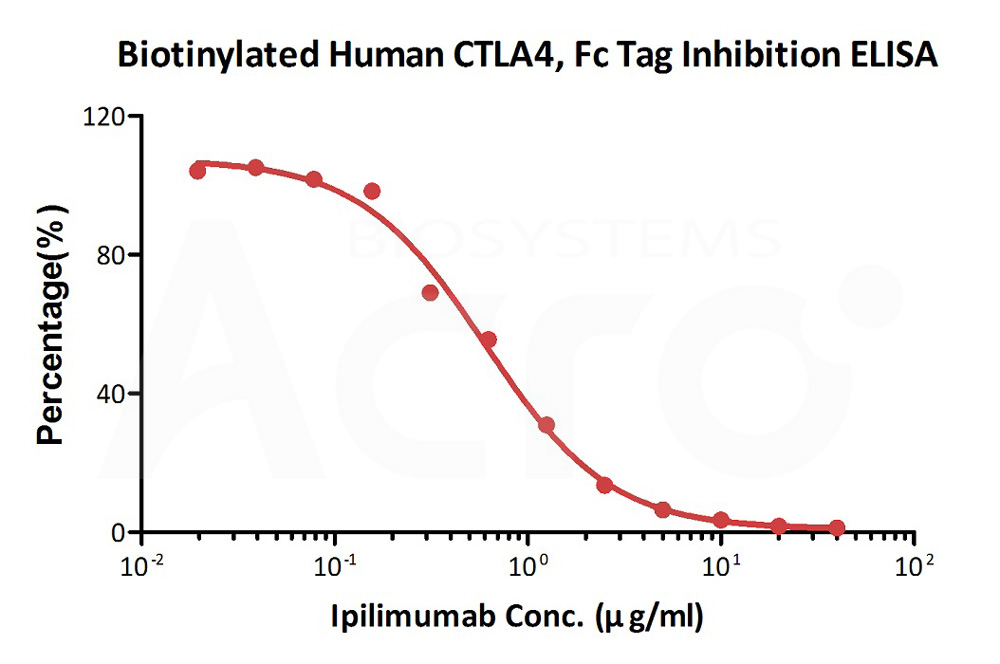
Serial dilutions of Ipilimumab neutralizing antibody (1:2 serial dilutions, from 40 μg/mL to 0.019 μg/mL) were added into Human B7-2, Fc Tag (Cat. No. CD6-H5257): Biotinylated Human CTLA-4, Fc,Avitag (Cat. No. CT4-H82F3) binding reactions. The assay was performed according to the above described protocol. Background was subtracted from data points before curve fitting.
Protocol
 +添加评论
+添加评论背景(Background)
CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4) is also known as CD152 (Cluster of differentiation 152), is a protein receptor that downregulates the immune system. CTLA4 is a member of the immunoglobulin superfamily, which is expressed on the surface of Helper T cells and transmits an inhibitory signal to T cells. The protein contains an extracellular V domain, a transmembrane domain, and a cytoplasmic tail. Alternate splice variants, encoding different isoforms. CTLA4 is similar to the T-cell co-stimulatory protein, CD28, and both molecules bind to CD80 and CD86, also called B7-1 and B7-2 respectively, on antigen-presenting cells. CTLA4 transmits an inhibitory signal to T cells, whereas CD28 transmits a stimulatory signal. Intracellular CTLA4 is also found in regulatory T cells and may be important to their function. Fusion proteins of CTLA4 and antibodies (CTLA4-Ig) have been used in clinical trials for rheumatoid arthritis.























































 膜杰作
膜杰作 Star Staining
Star Staining

















