Biomarkers of cell cycle arrest, microcirculation dysfunction, and inflammation in the prediction of SA-AKIZhang, Yang, Li
et alSci Rep (2025) 15 (1), 8023
Abstract: Sepsis-associated acute kidney injury (SA-AKI) is a severe complication in critically ill patients, with a complex pathogenesis involving in cell cycle arrest, microcirculatory dysfunction, and inflammation. Current diagnostic strategies remain suboptimal. Therefore, this study aimed to evaluate pathophysiology-based biomarkers and develop an improved predictive model for SA-AKI. The prospective observational study was conducted, enrolling 26 healthy individuals and 96 sepsis patients from Peking University Third Hospital. Clinical and laboratory data were collected, and patients were monitored for AKI development within 72 h. Further, sepsis patients were categorized into SA-noAKI (n = 46) and SA-AKI (n = 50) groups. Novel biomarkers, including tissue inhibitor of metalloproteinase-2 (TIMP-2), insulin-like growth factor-binding protein-7 (IGFBP-7), and angiopoietin-2 (Ang-2), were measured in all participants. Among these sepsis patients, the SA-AKI incidence was 52.08% (50/96). Compared to SA-noAKI, the SA-AKI group had significantly higher levels of TIMP-2 (93.55 [79.36, 119.56] ng/mL), IGFBP-7 (27.8 [21.44, 37.29] ng/mL), TIMP-2×IGFBP-7 (2.91 [1.90, 3.55] (ng/mL)²/1000), and Ang-2 (10.61 [5.79, 14.57] ng/mL) (P < 0.05). Accordingly, logistic regression identified TIMP-2×IGFBP-7 (OR = 2.71), Ang-2 (OR = 1.19), and PCT (OR = 1.05) as independent risk factors. The ROC curve of the predictive model demonstrated superior early-stage accuracy (AUC = 0.898), which remained stable during internal validation (AUC = 0.899). Meanwhile, the nomogram exhibited that this model was characterized with excellent discrimination, calibration, and clinical performance. In general, TIMP-2×IGFBP-7, Ang-2 and PCT were the independent risk factors for SA-AKI, and the novel model based on the three indicators provided a more accurate and sensitive strategy for the early prediction of SA-AKI.© 2025. The Author(s).
Association between serum levels of insulin-like growth factor-binding proteins at admission and outcomes at 3 months after acute ischemic strokeZhu, Wang, Cui
et alAnn Med (2025) 57 (1), 2472867
Abstract: Insulin-like growth factor-binding proteins (IGFBPs) contribute to central nervous system development and may influence recovery after stroke. This study aimed to determine whether serum IGFBPs levels in acute ischemic stroke (AIS) patients are associated with 3-month outcomes.We retrospectively reviewed data from AIS patients admitted within 24 h after stroke onset, and who had been prospectively enrolled in the Chengdu Stroke Registry. Serum IGFBPs 4, 6 and 7 levels at admission were compared between patients experienced good outcome (modified Rankin Scale scores of 0-2) or poor outcome (scores of 3-6) at 3 months after stroke onset. Factors associated with good outcome were identified using logistic regression.Among 194 patients, 115 (59.3%) experienced good outcome at 3 months. Patients with good outcome showed significantly higher levels of all three IGFBPs at admission. Good outcome was independently associated with higher serum levels of IGFBP 4 (OR 1.013, 95% CI 1.005-1.020) and IGFBP 7 (OR 1.012, 95% CI 1.003-1.021) after adjustment for potential confounders. Adding either or both IGFBPs to a model based on conventional clinical factors significantly improved good outcome prediction, with net reclassification improvement of 41.9-54.5% and integrated discrimination improvement of 3.8-5.8%. The model containing both IGFBPs predicted good outcome with an area of 0.878 (95% CI 0.827-0.929) under the receiver operating characteristic curve.Higher serum IGFBPs 4 and 7 levels may be associated with greater probability of good outcome at 3 months after AIS.
Advances in the diagnosis of early biomarkers for acute kidney injury: a literature reviewYang, Chen, He
et alBMC Nephrol (2025) 26 (1), 115
Abstract: Acute kidney injury (AKI) is a critical condition with diverse manifestations and variable outcomes. Its diagnosis traditionally relies on delayed indicators such as serum creatinine and urine output, making early detection challenging. Early identification is essential to improving patient outcomes, driving the need for novel biomarkers. Recent advancements have identified promising biomarkers across various biological processes. Tubular injury markers, including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), and liver-type fatty acid-binding protein (L-FABP), offer insights into early tubular damage. Inflammatory and repair-associated biomarkers, such as interleukin-18 (IL-18), monocyte chemotactic protein-1 (MCP-1), osteopontin (OPN), and C-C motif chemokine ligand 14 (CCL14), reflect ongoing injury and recovery processes. Additionally, stress and repair markers like tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor-binding protein-7 (IGFBP-7), alongside filtration markers such as cystatin C (CysC) and proenkephalin (PenKid®) e.tal, further enhance diagnostic precision. Oxidative stress-related markers, including Superoxide Dismutase 1 (SOD1), also contribute valuable information. Emerging candidates, such as microRNAs, soluble urokinase plasminogen activator receptor (SuPAR), and chitinase-3-like protein 1 (CHI3L1), hold substantial promise for AKI detection and prognosis. This review summarizes the progress in AKI biomarker research, highlighting their clinical utility and exploring their potential to refine early diagnosis and management strategies. These findings offer a new perspective for integrating novel biomarkers into routine clinical practice, ultimately improving AKI care.© 2025. The Author(s).
Effects of oral administration of the probiotic Lactobacillus rhamnosus GG on the proteomic profiles of cerebrospinal fluid and immunoregulatory signaling in the hippocampus of adult male ratsLoupy, Dawud, Zambrano
et alNeuroimmunomodulation (2025)
Abstract: The microbiome-gut-brain axis, by modulating bidirectional immune, metabolic, and neural signaling pathways in the host, has emerged as a target for the prevention and treatment of psychiatric and neurological disorders. Oral administration of the probiotic bacterium Lactobacillus rhamnosus GG (LGG; ATCC 53103) exhibits anti-inflammatory effects, although the precise mechanisms by which LGG benefits host physiology and behavior are not known. The goal of this study was to explore the general effects of LGG on the cerebrospinal fluid (CSF) proteome and a biological signature of anti-inflammatory signaling in the central nervous system (CNS) of undisturbed, adult male rats.Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomics were conducted using CSF samples collected after 21 days of oral treatment with live LGG (3.34 x 107 colony-forming units (CFU)/mL in the drinking water (resulting in an estimated delivery of ~1.17 x 109 CFU/day/rat) or water vehicle. Gene enrichment analysis (using DAVID, v. 6.8) and protein-protein interactions (using STRING, v. 11) were used to explore physiological network changes in CSF. Real time reverse transcription polymerase chain reaction (real time RT-PCR) was performed to assess gene expression changes of anti-inflammatory cytokines in the hippocampus. Genes associated with anti-inflammatory signaling that were analyzed included Il10, Tgfb1, Il4, and IL-4-responsive genes, Cd200, Cd200r1, and Mrc1 (Cd206).Oral LGG administration altered the abundance of CSF proteins, increasing the abundance of five proteins (cochlin, NPTXR, reelin, Sez6l, and VPS13C) and decreasing the abundance of two proteins (CPQ, IGFBP-7) in the CSF. Simultaneously, LGG increased the expression of Il10 mRNA, encoding the anti-inflammatory cytokine interleukin 10, in the hippocampus.Oral LGG altered the abundance of CSF proteins associated with extracellular scaffolding, synaptic plasticity, and glutamatergic signaling. These data are consistent with the hypothesis that oral administration of LGG may improve memory and cognition, and may promote a physiological resilience to neurodegenerative disease, by increasing glutamatergic signaling and promoting an anti-inflammatory environment in the brain.The Author(s). Published by S. Karger AG, Basel.

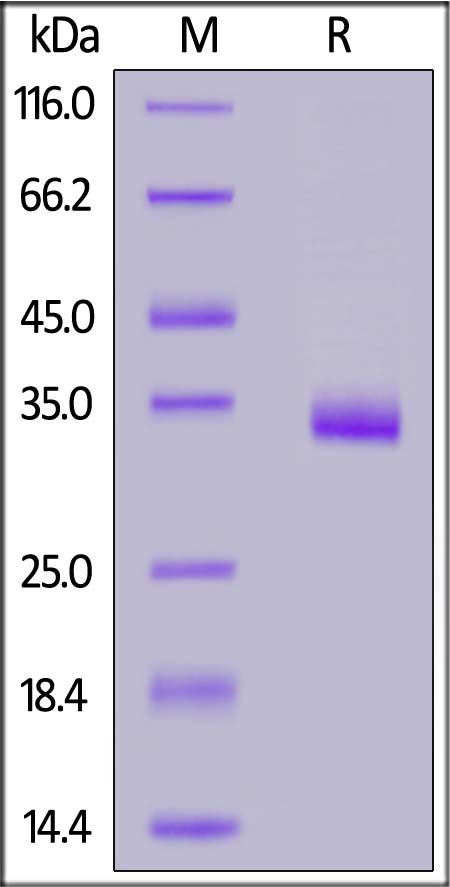
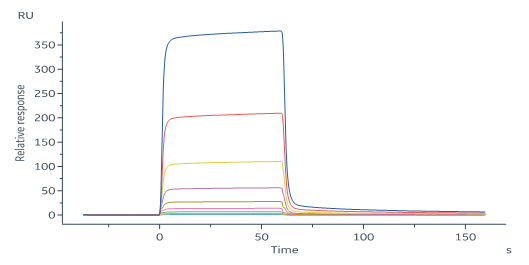
 +添加评论
+添加评论






















































 膜杰作
膜杰作 Star Staining
Star Staining
















