A synthetic heavy chain variable domain antibody library (VHL) provides highly functional antibodies with favorable developabilityPang, Wang, Yang
et alProtein Sci (2025) 34 (4), e70090
Abstract: Synthetic antibody libraries have been developed as an efficient source for the discovery of the heavy chain variable (VH) domain, which exhibits low immunogenicity, high tissue penetration, and diverse binding epitopes in therapeutic biopharmaceuticals. In this study, the human IGHV3-23*04 germline gene was chosen as the scaffold with a high expression level and favorable thermal stability. Amino acid diversity was introduced into the complementarity determining region 3 (CDR3) to exclude potential sequence liabilities. A library containing 2.6 × 1011 independent clones was successfully constructed. The receptor-binding domain (RBD) of the SARS-CoV-2 spike protein, interleukin-17A (IL17A), B-cell maturation antigen (BCMA), and G-protein coupled receptor family C group 5 member D (GPRC5D) were used as target antigens to screen and identify VHs. In each case, Thirty-one to fifty-five VHs were screened out. The VH-Fc antibodies showed superior affinities (as high as 4.6 nM) to the corresponding antigens but did not bind to antigen-irrelevant cell CHO-S. Furthermore, the anti-RBD and anti-IL17A VH-Fc antibodies showed strong functional activity in the receptor-blocking assays. The VH-Fc antibodies from the synthetic library exhibited favorable developability (thermal stability, colloidal stability, hydrophilicity, anti-aggregation ability, and no interaction with human IgGs). We demonstrated that high-affinity and highly functional VH domain antibodies were generated from the rationally designed library with desired physicochemical properties. This approach is generally universal to target any antigen and has significant potential to accelerate candidate selection.© 2025 The Protein Society.
Safety and activity of talquetamab in patients with relapsed or refractory multiple myeloma (MonumenTAL-1): a multicentre, open-label, phase 1-2 studyChari, Touzeau, Schinke
et alLancet Haematol (2025)
Abstract: Talquetamab is the first GPRC5D × CD3 bispecific antibody approved for relapsed or refractory multiple myeloma. In phase 1 of the MonumenTAL-1 study, initial results of subcutaneous talquetamab 0·4 mg/kg once a week and 0·8 mg/kg every 2 weeks showed preliminary clinical activity. We describe safety and activity results in patients treated with talquetamab, including patients who had received previous T-cell redirection therapy (TCR). This post-hoc analysis was done with more mature median follow-up to evaluate duration of response in patients treated with talquetamab 0·8 mg/kg every 2 weeks.MonumenTAL-1 is a multicentre, open-label, phase 1-2 study of talquetamab, phase 1 of which has previously been published. The 0·4 mg/kg once a week and 0·8 mg/kg every 2 weeks recommended subcutaneous doses identified in phase 1 were evaluated in phase 2 in patients who were 18 years of age or older, had at least three previous lines of therapy, had an Eastern Cooperative Oncology Group performance status of 0 to 2, and were naive or exposed to previous TCR. The primary endpoint was overall response rate assessed by independent review committee in all patients who received at least one dose of talquetamab. Safety was assessed in all patients who received at least one dose of talquetamab. This study was registered with ClinicalTrials.gov, NCT03399799 (phase 1) and NCT04634552 (phase 2).Between Jan 3, 2018, and Feb 20, 2023, 735 patients were screened across all phase 1-2 cohorts. Of these, 537 patients screened for inclusion were treated across phase 1 and 2 cohorts, of whom 1983 (27%) patients were excluded from the study, most commonly due to not meeting eligibility criteria or not having measurable disease. As of Oct 11, 2023, 375 patients had received recommended talquetamab doses across three groups: 143 (0·4 mg/kg once a week group) and 154 (0·8 mg/kg every 2 weeks group) TCR-naive patients and 78 with previous TCR who received either recommended dose (previous TCR group). 217 (58%) of 375 patients were male and 158 (42%) were female. 325 (87%) of 375 patients were White and 32 (9%) patients were Black. Median follow-up was 25·6 months (IQR 8·5-25·9) in the 0·4 mg/kg once a week group, 19·4 months (9·2-20·7) in the 0·8 mg/kg every 2 weeks group, and 16·8 months (7·6-18·7) in the previous TCR group. Overall response rate was 74% (106 of 143 patients, 95% CI 66-81) in the 0·4 mg/kg once a week group, 69% (107 of 154 patients, 62-77) in the 0·8 mg/kg every 2 weeks group, and 67% (52 of 78 patients, 55-77) in the previous TCR group. Most common adverse events in the 0·4 mg/kg once a week, 0·8 mg/kg every 2 weeks, and previous TCR groups were cytokine release syndrome (113 [79%] of 143 patients, 115 [75%] of 154 patients, and 57 [73%] of 78 patients), taste changes (103 [72%], 110 [71%], and 59 [76%]), and infections (85 [59%], 105 [68%], and 59 [76%]). Most common grade 3-4 adverse events were neutropenia (44 [31%], 33 [21%], and 37 [47%]), anaemia (45 [31%], 40 [26%], and 21 [27%]), and lymphopenia (37 [26%], 40 [26%], and 13 [17%]). Fatal adverse events occurred in five patients in the 0·4 mg/kg once a week group, seven patients in the 0·8 mg/kg every 2 weeks group, and no patients in the previous TCR group; none were related to treatment.Talquetamab continued to demonstrate high overall response rates in heavily pretreated patients with relapsed or refractory multiple myeloma with longer follow-up in this post-hoc analysis. Overall response rate was promising in patients with previous TCR, including therapies targeting BCMA. On-target, off-tumour adverse events were common but led to few treatment discontinuations.Janssen.Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Genetic and epigenetic mechanisms of GPRC5D loss after anti-GPRC5D CAR T-cell therapy in multiple myelomaMa, Xia, Zhang
et alBlood (2025)
Abstract: G protein-coupled receptor, class C, group 5, member D (GPRC5D) has emerged as a novel target for chimeric antigen receptor (CAR) T-cell therapy, demonstrating promising efficacy in multiple myeloma (MM). However, disease relapse is still common, and the mechanism of resistance remains poorly understood. In this study, we conducted whole-genome sequencing (WGS) and whole-genome bisulfite sequencing (WGBS) on MM samples from 10 patients who relapsed after GPRC5D CAR T-cell therapy. Among these patients, 8 had GPRC5D loss, while 2 presented mixed expression (GPRC5D+/-). Genetic alterations were identified in three cases: one had a homozygous deletion in the GPRC5D gene, another had a biallelic loss in the regulatory regions of GPRC5D, and the third had homozygous deletions in both TNFRSF17 and GPRC5D after sequential anti-BCMA and anti-GPRC5D CAR T-cell therapies. No genetic changes were detected at GPRC5D locus in the remaining 7 cases. However, multiple hypermethylation sites were present in the transcriptional regulatory elements of the GPRC5D gene in 5 post-treatment MM samples. In MM cell lines, GPRC5D expression was inversely correlated with methylation levels in its regulatory regions. Furthermore, azacitidine treatment induced GPRC5D mRNA and protein expression in hypermethylated MM cell lines. Our findings highlight that biallelic genetic inactivation and hypermethylation-driven epigenetic silencing are key mechanisms contributing to GPRC5D loss and treatment resistance.Copyright © 2025 American Society of Hematology.
Memory-like NK cell differentiation, inhibitory NKG2A blockade, and improved recognition via antibody or CAR engineering combine to enhance NK cell attack against multiple myelomaZhou, Marin, Afrin
et alJ Immunol (2025) 214 (1), 1-11
Abstract: Natural killer (NK) cells are a promising approach for cellular cancer immunotherapy and are being investigated to treat patients with multiple myeloma (MM). We found that MM patient blood NK cell frequencies were normal with increased activating receptors and cytotoxic granules, without evidence of functional exhaustion. Despite this activated state, MM target cells were resistant to conventional NK cells by unclear mechanisms. Memory-like (ML) NK cells are generated after brief activation via the interleukin (IL)-12, IL-15, and IL-18 receptors and exhibit multiple enhanced antitumor properties. ML NK cell differentiation improved healthy donor and MM patient NK cell responses against MM target cells, in vitro and in vivo in immunodeficient murine xenograft models. Moreover, incorporating NKG2A checkpoint blockade to overcome HLA-E-induced inhibition further enhanced ML NK cell responses against MM in vitro and in vivo. Because activating receptor recognition of MM by ML NK cells was inadequate, strategies to improve this were investigated. Utilizing anti-SLAMF7 monoclonal antibody (elotuzumab) or anti-BCMA chimeric antigen receptors resulted in robust increases in ML NK cell functional responses against MM. In summary, ML differentiation enhances NK cell attack against myeloma, and combination with approaches to block inhibitory checkpoints and promote MM-specific activation are promising translational NK cell strategies for MM immunotherapy.© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.

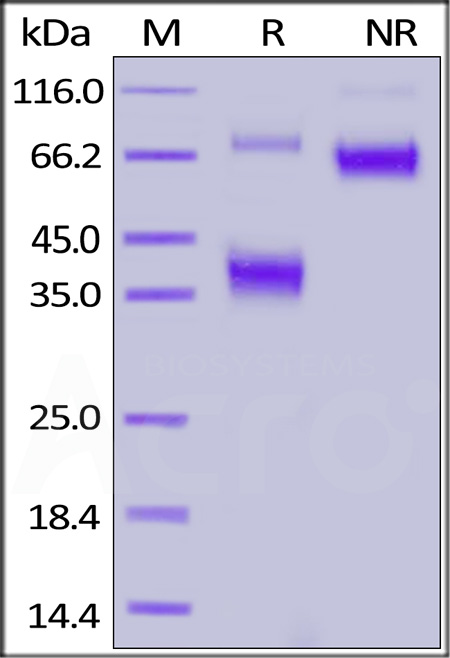
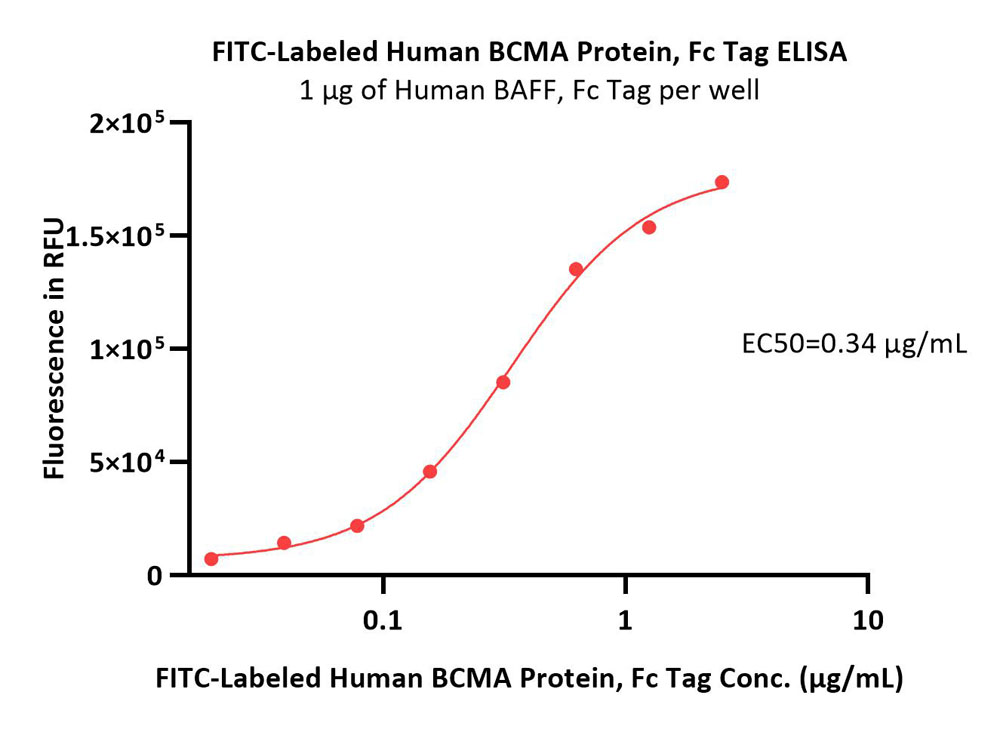
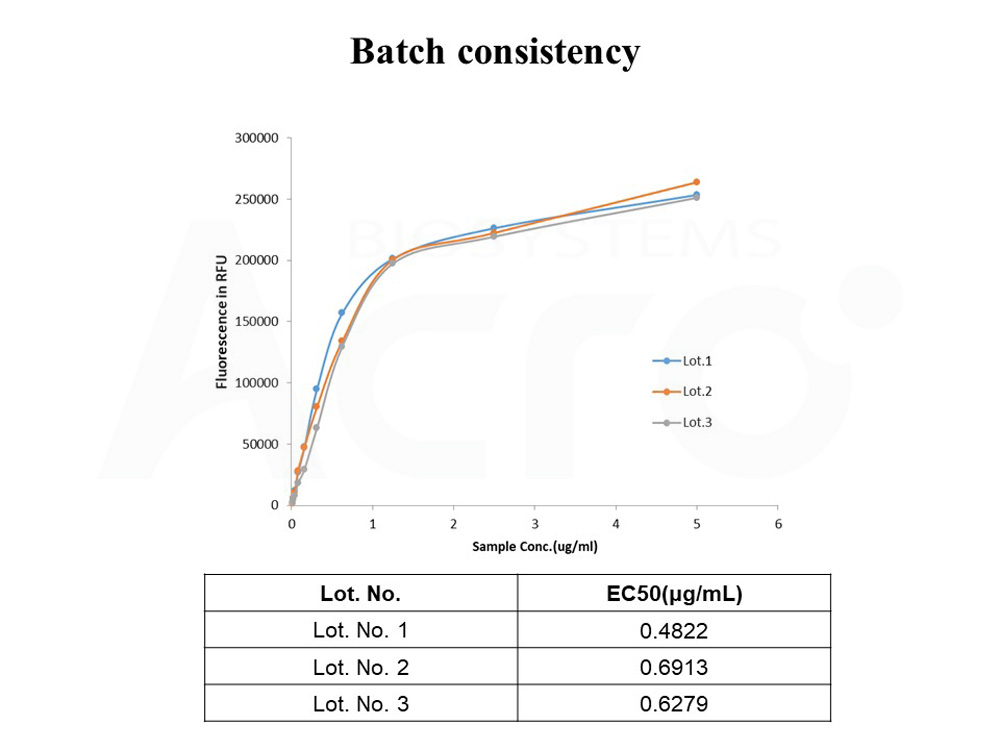
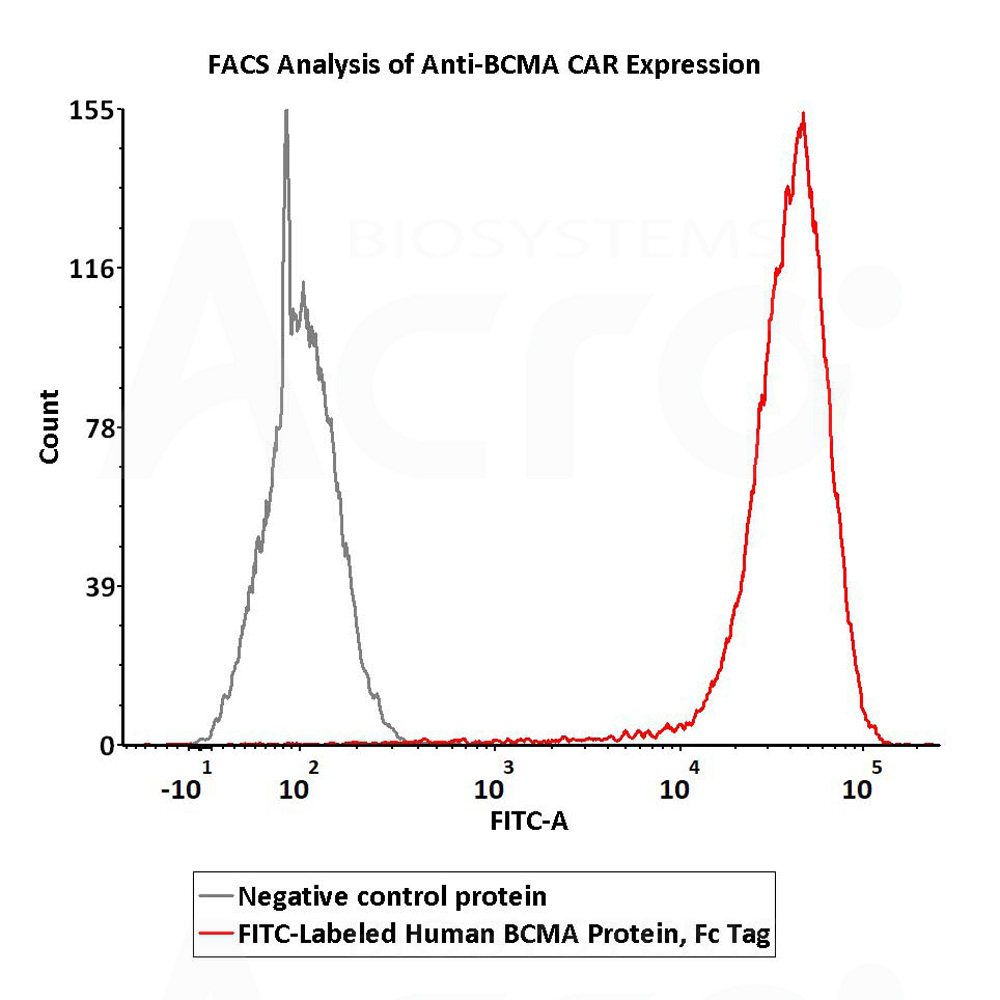
 +添加评论
+添加评论























































 膜杰作
膜杰作 Star Staining
Star Staining

















