分子别名(Synonym)
CD47,MER6,IAP,OA3
表达区间及表达系统(Source)
Biotinylated Human CD47, His,Avitag (CD7-H82E9) is expressed from human 293 cells (HEK293). It contains AA Gln 19 - Pro 139 (Accession # Q08722-3).
Predicted N-terminus: Gln 19
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries a polyhistidine tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 16.9 kDa. The protein migrates as 32-44 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Labeling)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 24 months in lyophilized state;
- -70°C for 12 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
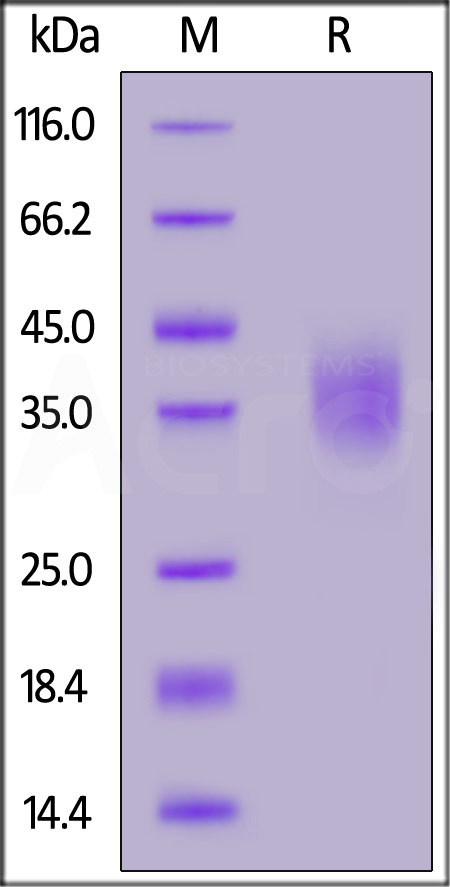
Biotinylated Human CD47, His,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
SEC-MALS
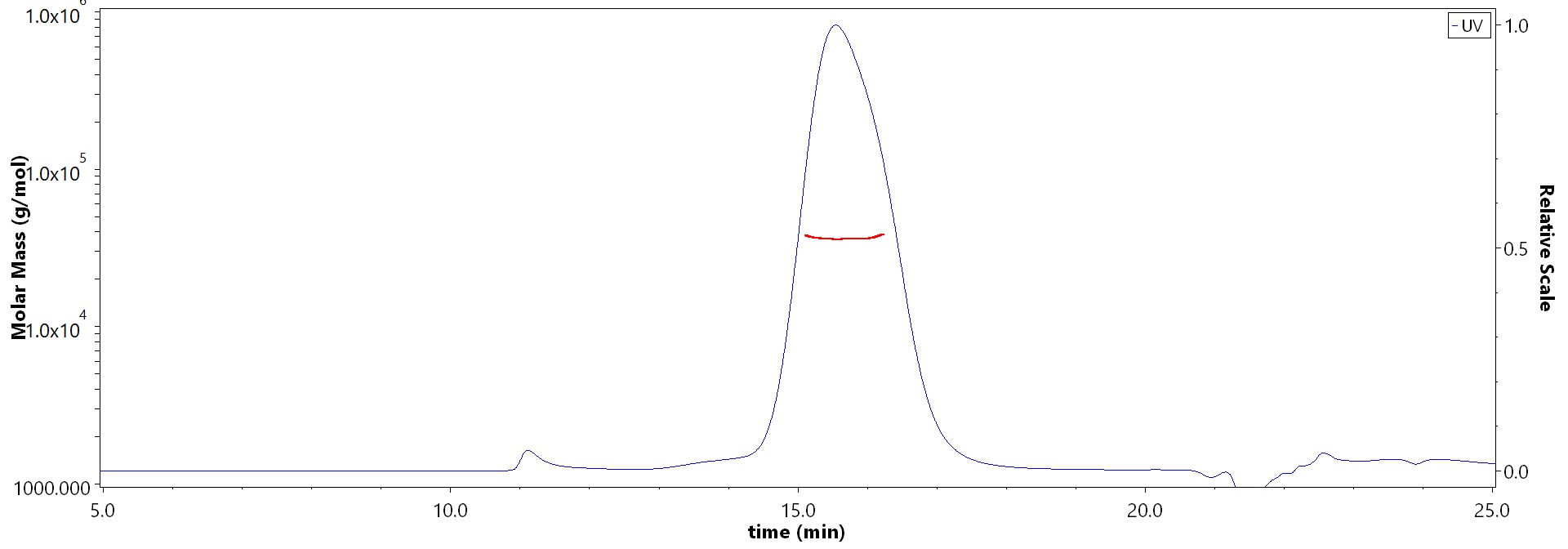
The purity of Biotinylated Human CD47, His,Avitag (Cat. No. CD7-H82E9) is more than 90% and the molecular weight of this protein is around 28-38 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA

Immobilized Monoclonal Anti-Human CD47 Antibody, Human IgG4 at 2 μg/mL (100 μL/well) can bind Biotinylated Human CD47, His,Avitag (Cat. No. CD7-H82E9) with a linear range of 0.1-3 ng/mL (QC tested).
Protocol

Immobilized Human SIRP alpha, Fc Tag (Cat. No. SIA-H5251) at 2 μg/mL (100 μL/well) can bind Biotinylated Human CD47, His,Avitag (Cat. No. CD7-H82E9) with a linear range of 2-25 ng/mL (Routinely tested).
Protocol
活性(Bioactivity)-FACS
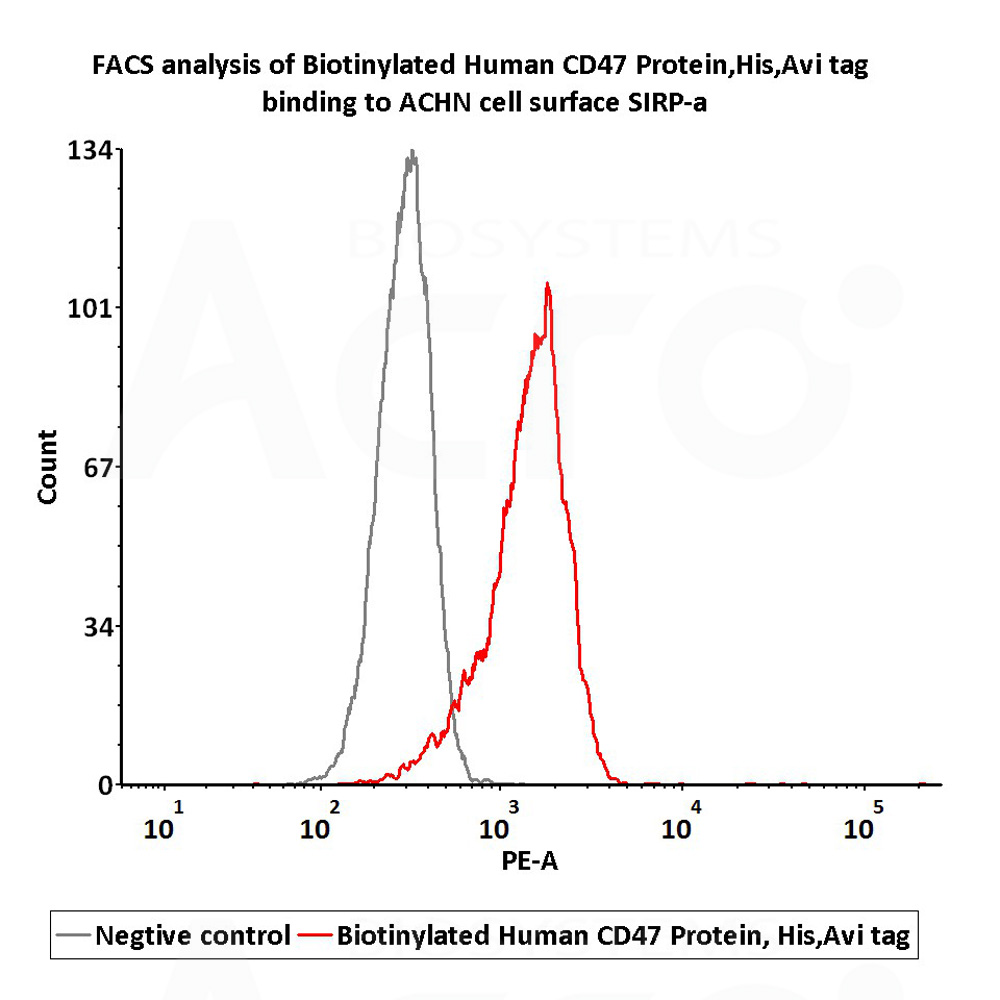
FACS assay shows that Biotinylated Human CD47, His,Avitag (Cat. No. CD7-H82E9) can bind to ACHN cell expressing human SIRP-a. The concentration of CD47 used is 10 μg/mL (Routinely tested).
Protocol

FACS analysis shows that the binding of Human CD47 to ACHN expressing SIRP-a was inhibited by increasing concentration of neutralizing SIRP-a antibody. The concentration of Human CD47 used is 20 μg/mL. IC50=9.334 μg/mL
Protocol
 +添加评论
+添加评论
- 156XXXXXXX8
- 用于开展抗体对SIRP-α和CD47之间结合的阻断实验,流式上生物素标记的CD47蛋白在2微克/毫升的情况下即可以与SIRP-α具有极高的亲和力。
- 2021-8-19

- 183XXXXXXX9
- 利用生物素-链霉亲和素系统的高特异性和高灵敏度的结合特性,我们将该生物素化蛋白与链霉亲和素相关产品联合应用于ELISA实验,与非生物素体系相比,检测速度更快、结合能力更强、非特异背景更低。通过分析近一年的检测结果,均未出现数据异常的情况,所有的检测也能满足检测方法设定的可接受标准。
- 2023-6-28

- 158XXXXXXX0
- 抗体是实验室最常用科研试剂之一。抗体特异性不好、抗体稳定性不佳,或者应用效果不好都会影响结果的输出,最终还会导致实验延期。不好的抗体会导致实验结果缺乏可重复性,造成时间和金钱浪费。ACRO抗体稳定性好,得到了出色的结果。
- 2022-6-17
背景(Background)
Leukocyte surface antigen CD47 is also known as Antigenic surface determinant protein OA3, Integrin-associated protein (IAP) and Protein MER6. CD47 contains 1 Ig-like V-type (immunoglobulin-like) domain. CD47 is very broadly distributed on normal adult tissues. CD47 has a role in both cell adhesion by acting as an adhesion receptor for THBS1 on platelets, and in the modulation of integrins and plays an important role in memory formation and synaptic plasticity in the hippocampus by similarity. CD47 is the receptor for SIRPA, binding to which prevents maturation of immature dendritic cells and inhibits cytokine production by mature dendritic cells. CD47 Interaction with SIRPG mediates cell-cell adhesion, enhances superantigen-dependent T-cell-mediated proliferation and costimulates T-cell activation.























































 膜杰作
膜杰作 Star Staining
Star Staining
















