分子别名(Synonym)
IL4,BCGF1,BSF1
表达区间及表达系统(Source)
Human IL-4, premium grade (IL4-H4218) is expressed from human 293 cells (HEK293). It contains AA His 25 - Ser 153 (Accession # P05112-1).
Predicted N-terminus: His 25
It is produced under our rigorous quality control system that incorporates a comprehensive set of tests including sterility and endotoxin tests. Product performance is carefully validated and tested for compatibility for cell culture use or any other applications in the early preclinical stage.
GMP-L04H26 is the GMP version of this IL4-H4218. These two proteins display indistinguishable performance profiles, thereby ensuring a seamless transition for end users from early preclinical stag to later clinical phases.
Request for sequence
蛋白结构(Molecular Characterization)

This protein carries no "tag".
The protein has a calculated MW of 15.0 kDa. The protein migrates as 19 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
内毒素(Endotoxin)
Less than 0.01 EU per μg by the LAL method.
宿主蛋白残留(Host Cell Protein)
<0.5 ng/µg of protein tested by ELISA.
宿主核酸残留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
无菌(Sterility)
Negative
支原体(Mycoplasma)
Negative.
纯度(Purity)
>95% as determined by SDS-PAGE.
>95% as determined by SEC-HPLC.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
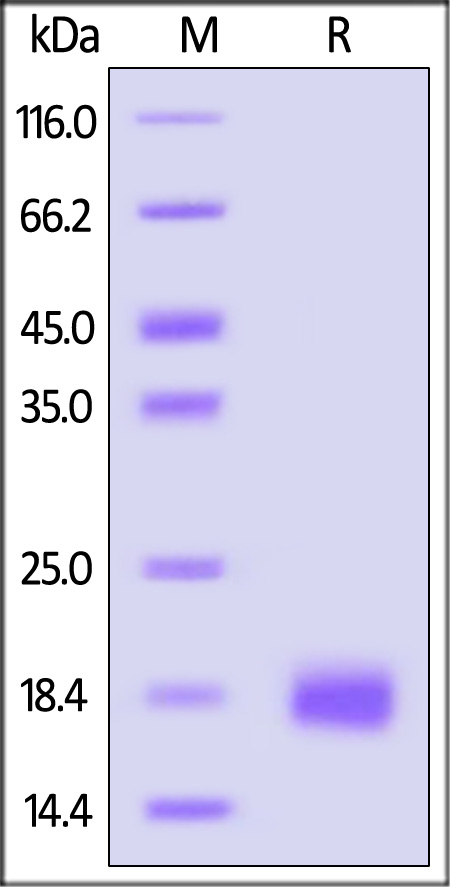
Human IL-4, premium grade on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95% (With Star Ribbon Pre-stained Protein Marker).
SEC-HPLC
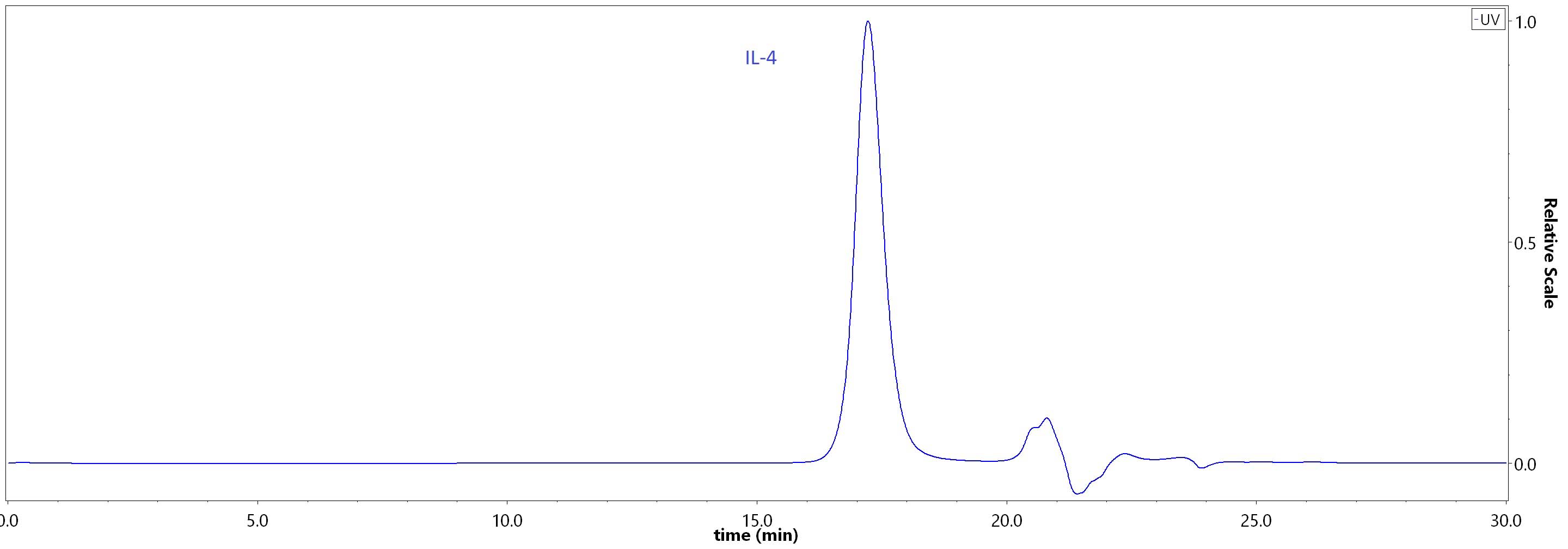
The purity of Human IL-4, premium grade (Cat. No. IL4-H4218) was greater than 95% as determined by SEC-HPLC.
活性(Bioactivity)-CELL BASE
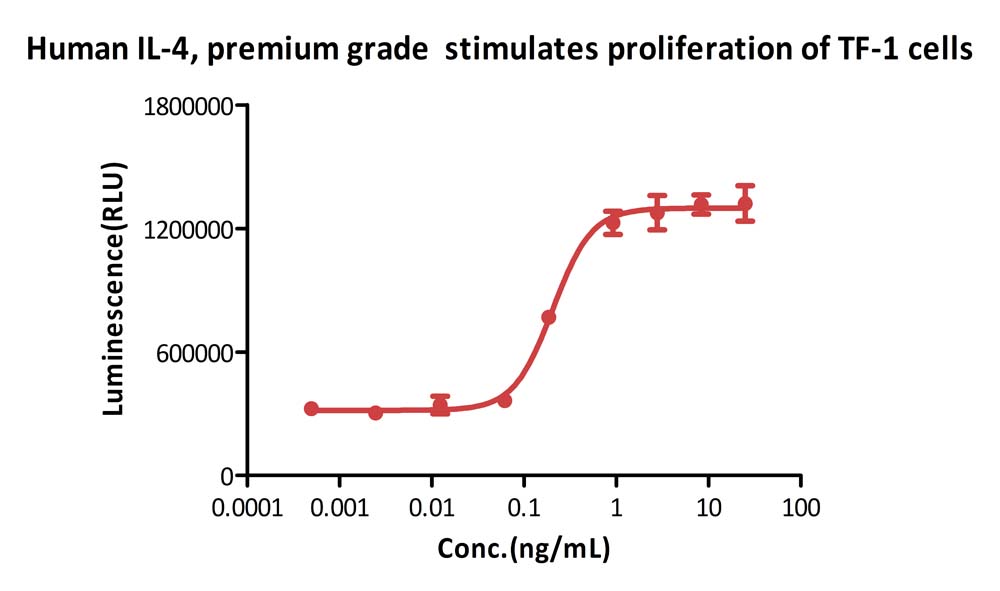
Human IL-4, premium grade (Cat. No. IL4-H4218) stimulates proliferation of TF-1 human erythroleukemic cell line. The specific activity of Human IL-4, premium grade is > 1.20×10^7 IU/mg, which is calibrated against human IL-4 WHO International Standard (NIBSC code: 88/656) (QC tested).
Protocol
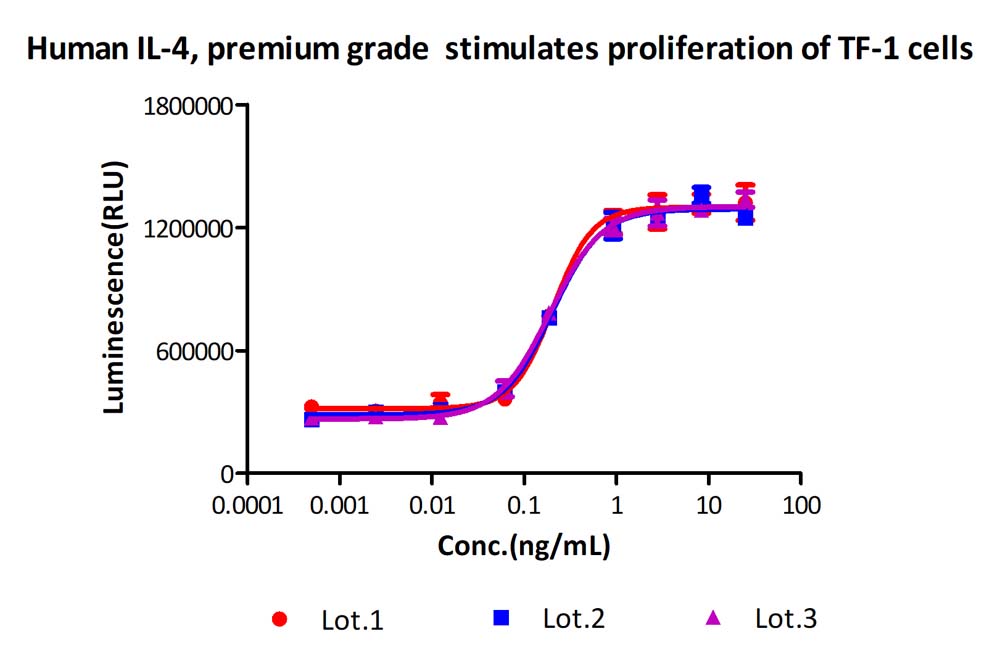
Activity of three different production batches of Human IL-4 Protein, premium grade (Cat. No. IL4-H4218).
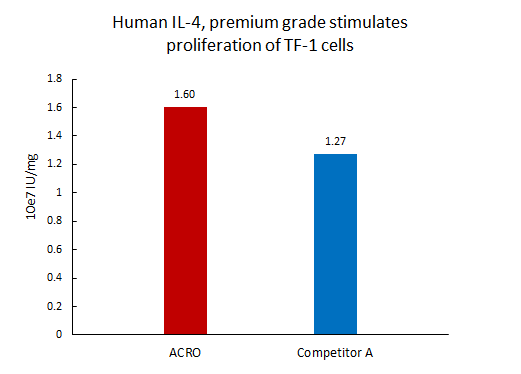
The activity of Human IL-4 Protein, premium grade (Cat. No. IL4-H4218) was higher than other competing products.
活性(Bioactivity)-ELISA
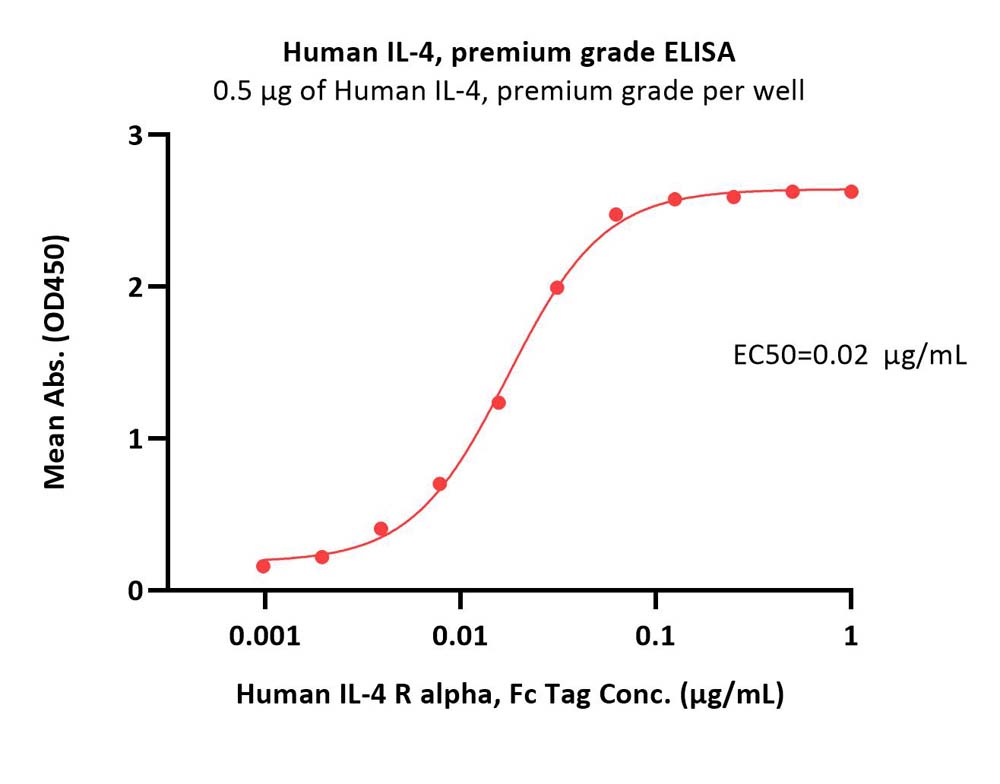
Immobilized Human IL-4, premium grade (Cat. No. IL4-H4218) at 5 μg/mL (100 μL/well) can bind Human IL-4 R alpha, Fc Tag (Cat. No. ILR-H5253) with a linear range of 0.001-0.031 μg/mL (QC tested).
Protocol
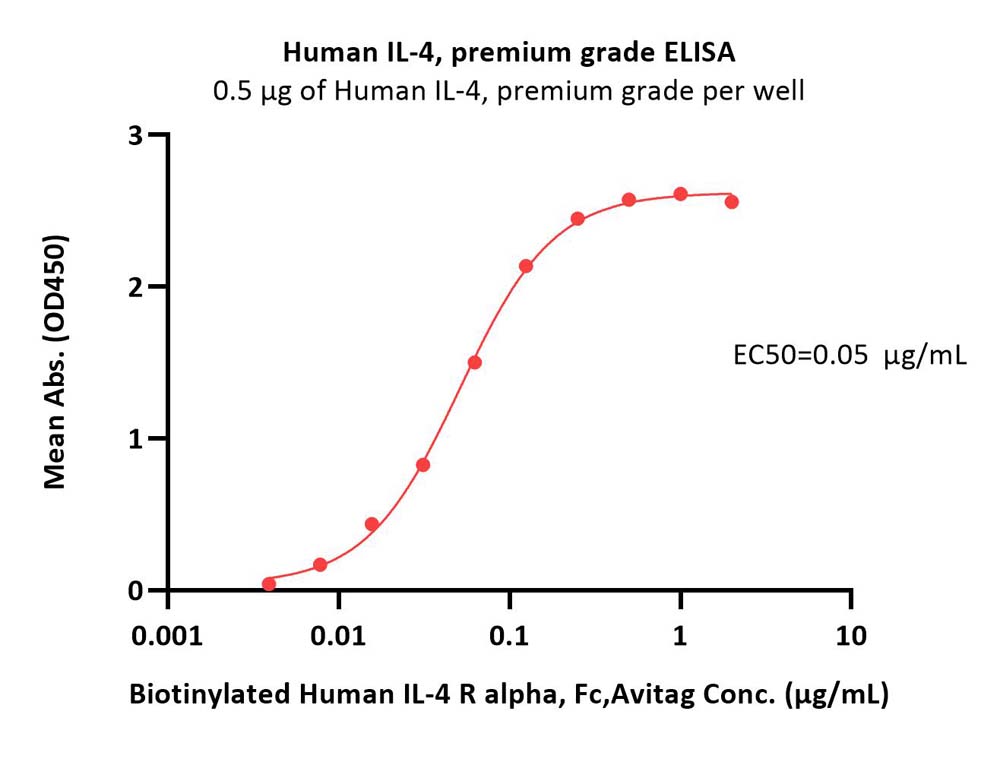
Immobilized Human IL-4, premium grade (Cat. No. IL4-H4218) at 5 μg/mL (100 μL/well) can bind Biotinylated Human IL-4 R alpha, Fc,Avitag (Cat. No. ILR-H82F4) with a linear range of 0.008-0.125 μg/mL (Routinely tested).
Protocol
稳定性(Stability)
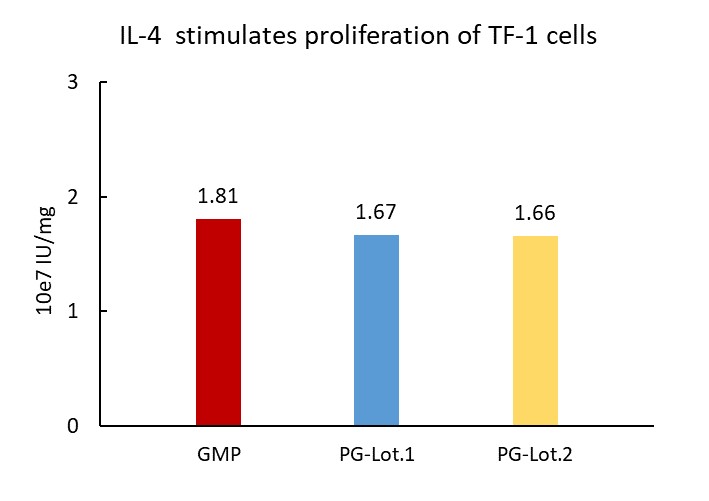
The Cell based assay shows batch-to-batch consistency between Acro's GMP and PG IL-4.
 +添加评论
+添加评论
- 156XXXXXXX8
- 购买该蛋白用于巨噬细胞诱导,开展巨噬细胞相关功能性验证实验,在添加M-CSF和IL-4的情况下,可以诱导PBMC和THP1细胞分化成为具有免疫抑制作用的M2型巨噬细胞。
- 2021-8-19

- 187XXXXXXX9
- 这个我们用来做ELISA的,效果还是比较不错的,梯度很好,主要和我们的抗体匹配度比较高,实验做起来挺轻松的,这个质量还是比较稳定的,发货也快,比较适合科研
 >
>- 2022-11-18

- 156XXXXXXX8
- 该多效细胞因子IL4主要用于体外细胞诱导协同GMCSF作用,成功诱导获得的细胞性能稳定,目的细胞能较好的组成型表达特异性表面标记物,为后续实验提供有力支撑。
 >
>- 2023-9-6
背景(Background)
Interleukin-4, is a cytokine that induces differentiation of naive helper T cells (Th0 cells to Th2 cells). In the presence of IL-4 and IL-13, cytokines that are produced in a Th-2 type response, particularly during allergy and parasitic infections, macrophages become differentially activated, And this cytokine is a ligand for interleukin 4 receptor. The interleukin 4 receptor also binds to IL13, which may contribute to many overlapping functions of this cytokine and IL13. STAT6, a signal transducer and activator of transcription, has been shown to play a central role in mediating the immune regulatory signal of this cytokine. Recently, researcher found that the cytokine IL-4 plays a key role in development of innate CD8+ T cells in the thymus of several gene-deficient mouse strains, including Itk, KLF2, CBP and Id3, without previous exposure to antigen.























































 膜杰作
膜杰作 Star Staining
Star Staining
















