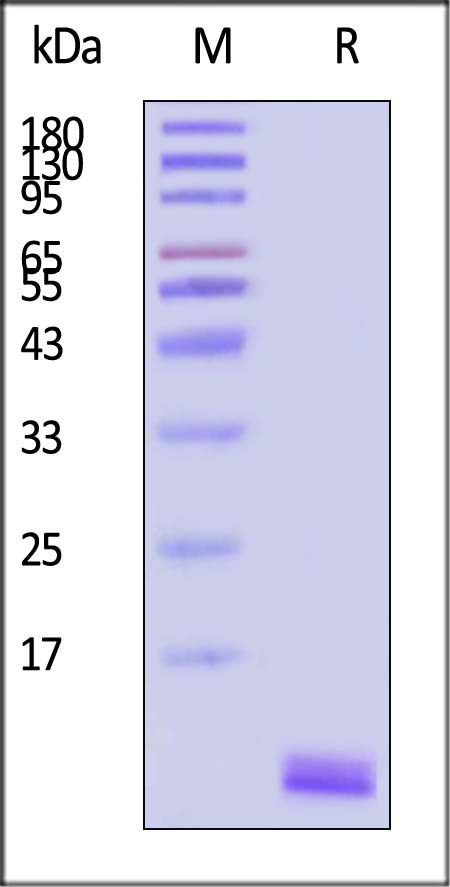
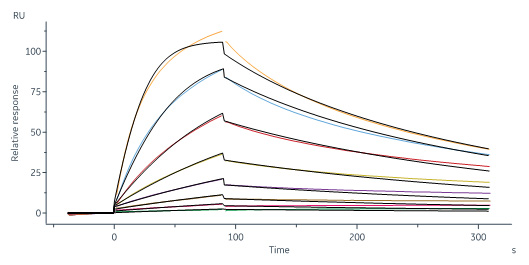
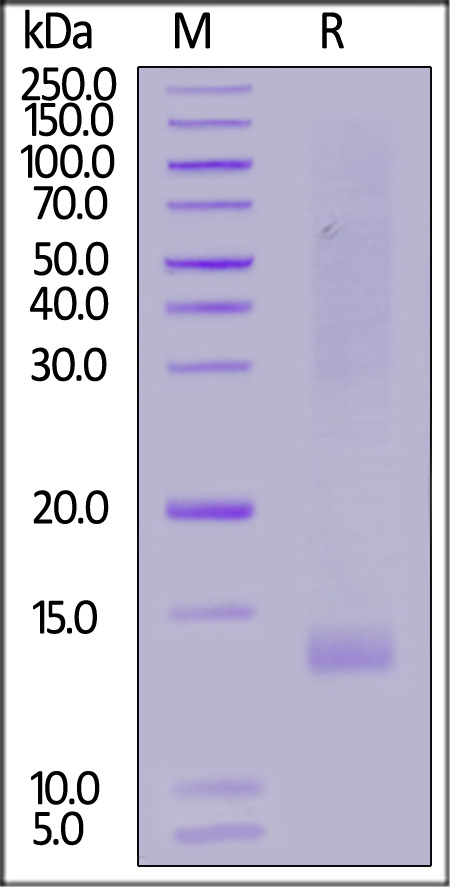
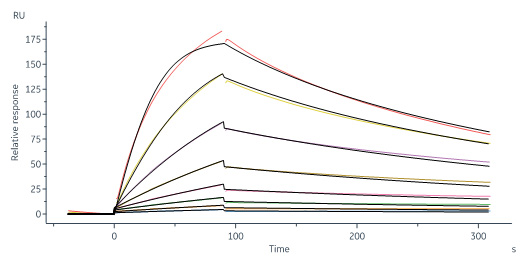
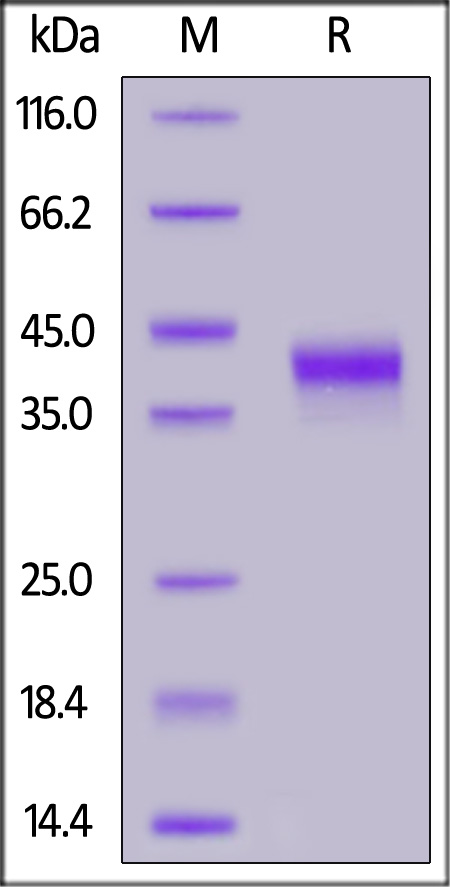
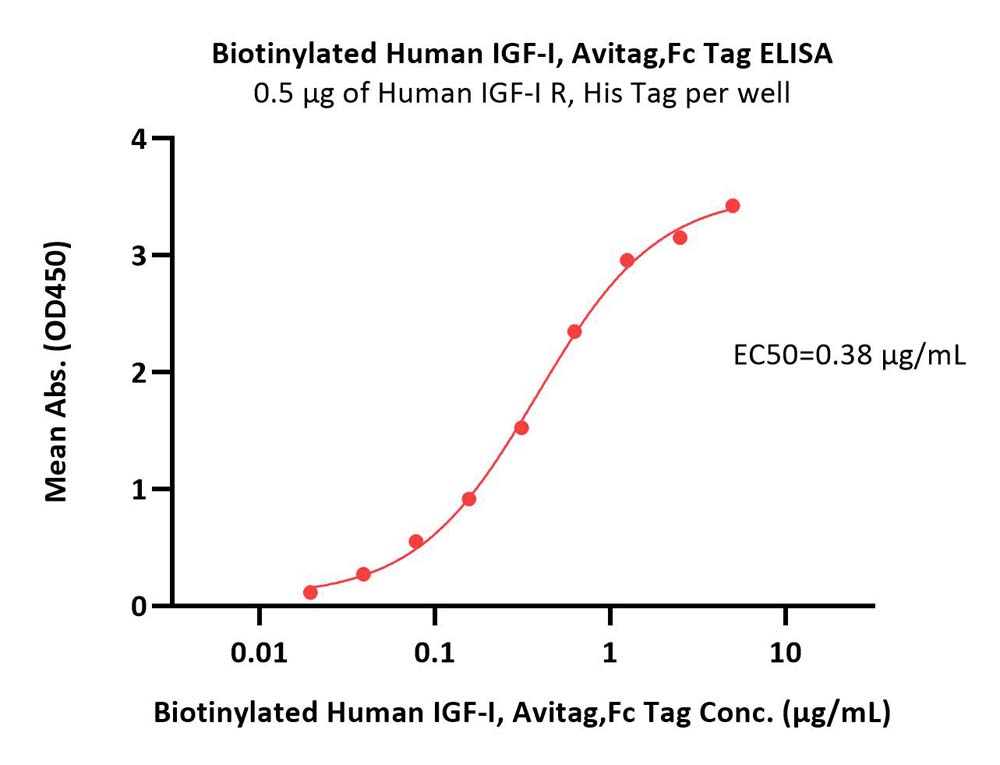
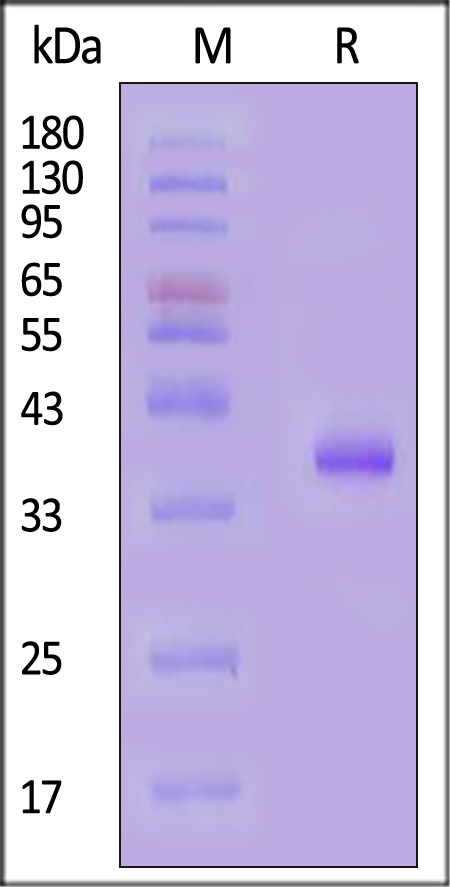
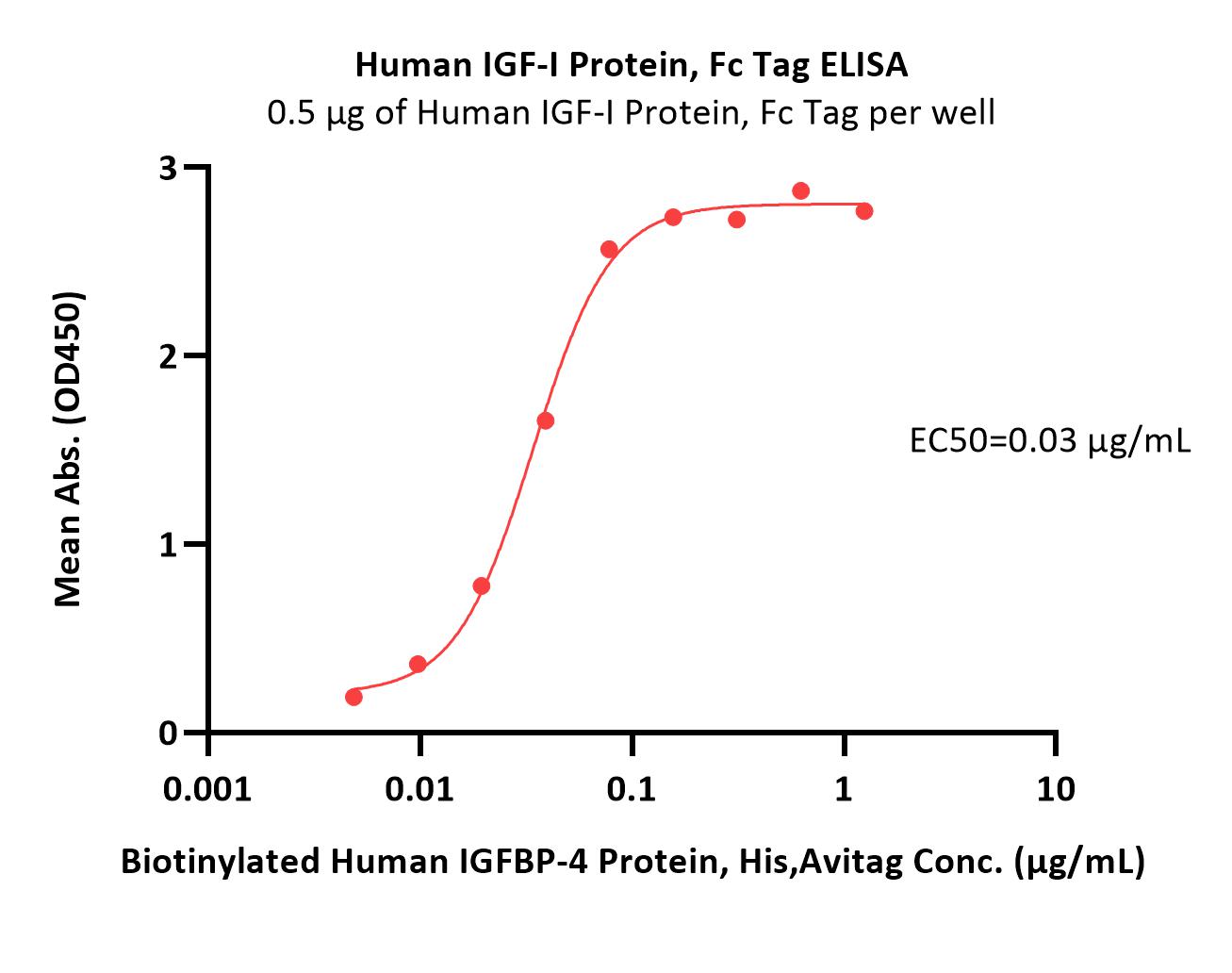
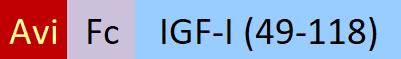IGF-I分子别名
IGF-I,IGF1A,somatomedin C,MGF
IGF-I分子背景
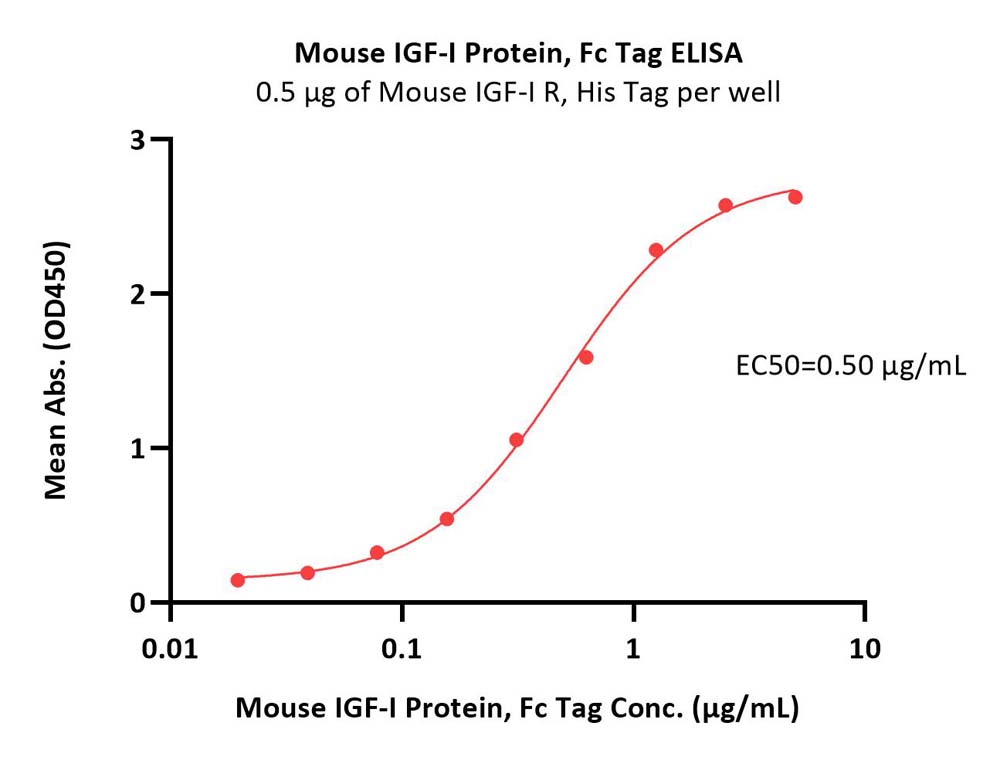
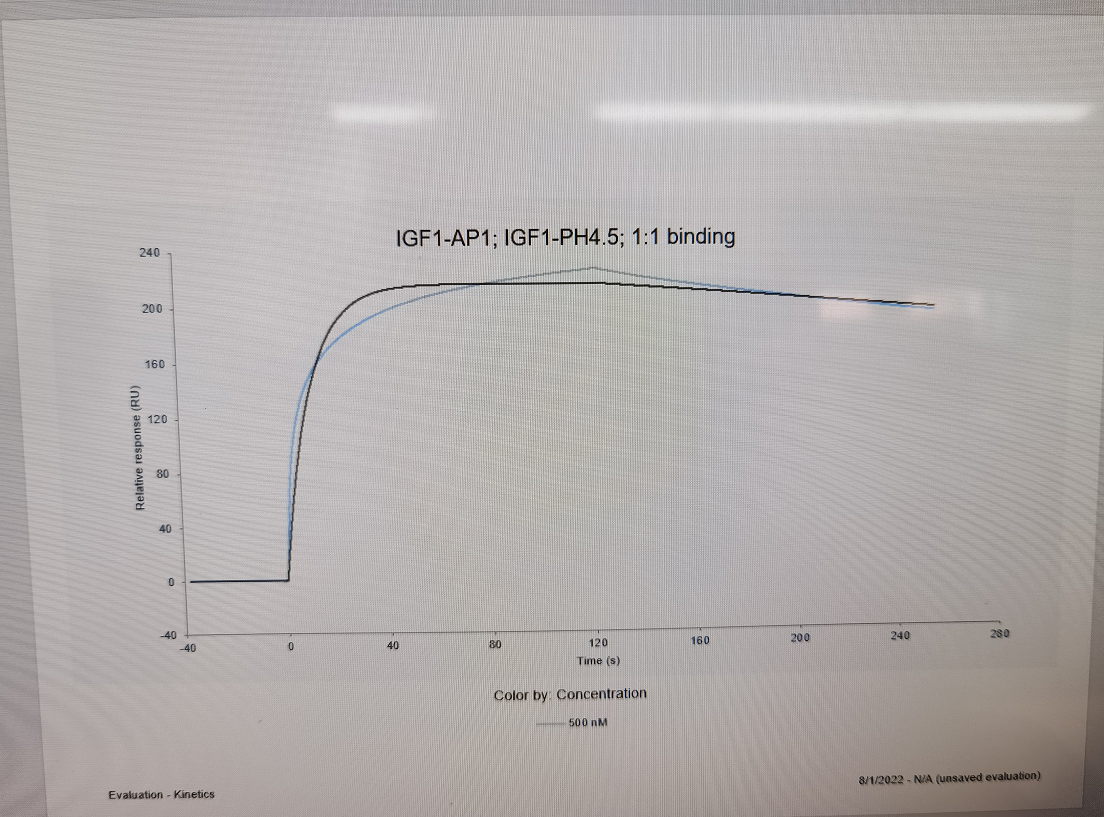
Insulin-like growth factor 1 (IGF-1) is also known as somatomedin C, IGF1A, IGFI, sulfation factor, and is a hormone similar in molecular structure to insulin. It plays an important role in childhood growth and continues to have anabolic effects in adults. A synthetic analog of IGF-1, mecasermin is used for the treatment of growth failure. IGF-1 consists of 70 amino acids in a single chain with three intramolecular disulfide bridges. IGF-1 has a molecular weight of 7649 daltons. IGF-1 is produced primarily by the liver as an endocrine hormone as well as in target tissues in a paracrine/autocrine fashion. IGF-1 binds to at least two cell surface receptors: the Insulin-like growth factor 1 receptor, abbreviated as "IGF1R", and the insulin receptor. The IGF-1 receptor seems to be the "physiologic" receptor - it binds IGF-1 at significantly higher affinity than the IGF-1 that is bound to the insulin receptor. Like the insulin receptor, the IGF-1 receptor is a receptor tyrosine kinase - meaning it signals by causing the addition of a phosphate molecule on particular tyrosines. Its primary action is mediated by binding to its specific receptor IGF1R, present on many cell types in many tissues. Binding to the IGF1R, a receptor tyrosine kinase, initiates intracellular signaling; IGF-1 is one of the most potent natural activators of the AKT signaling pathway, a stimulator of cell growth and proliferation, and a potent inhibitor of programmed cell death. Insulin-like growth factor 1 has been shown to bind and interact with all the IGF-1 Binding Proteins (IGFBPs), of which there are six (IGFBP1-6). Specific references are provided for interactions with IGFBP3, IGFBP4 and IGFBP7.






















 Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!
Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!































 膜杰作
膜杰作 Star Staining
Star Staining