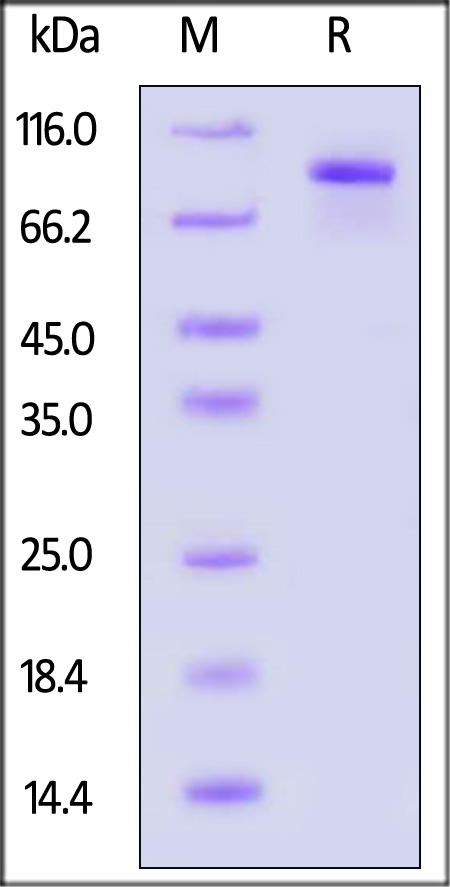
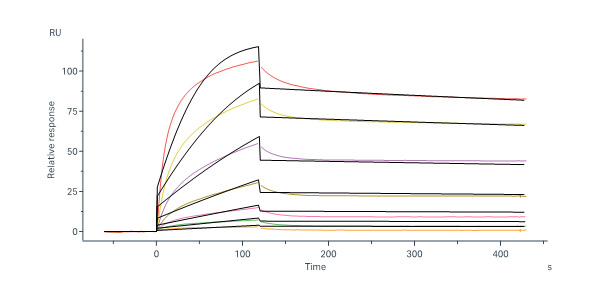
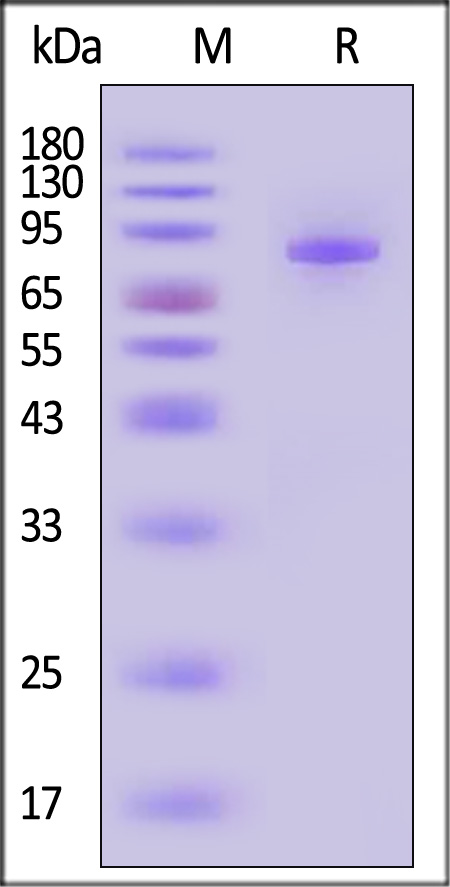
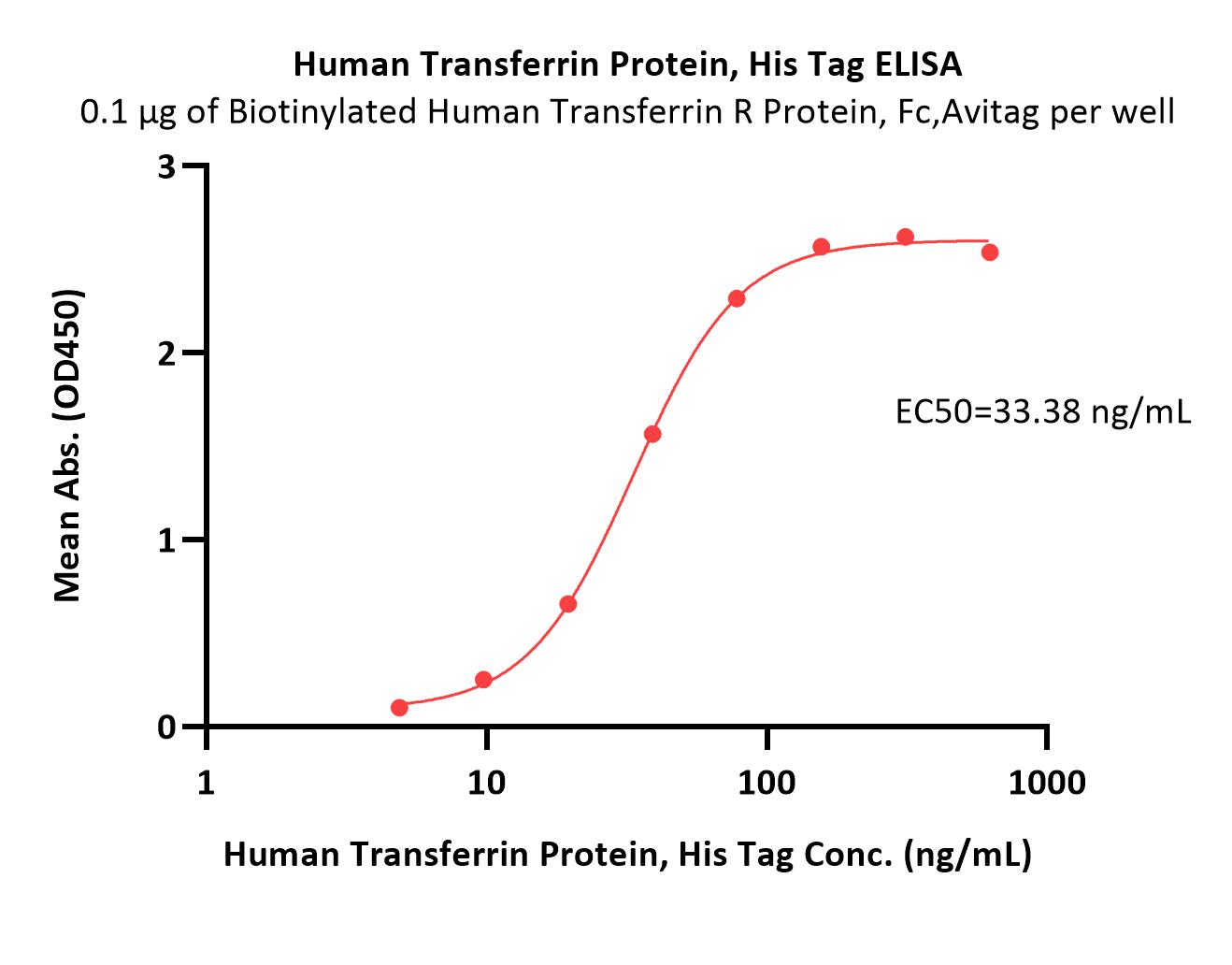
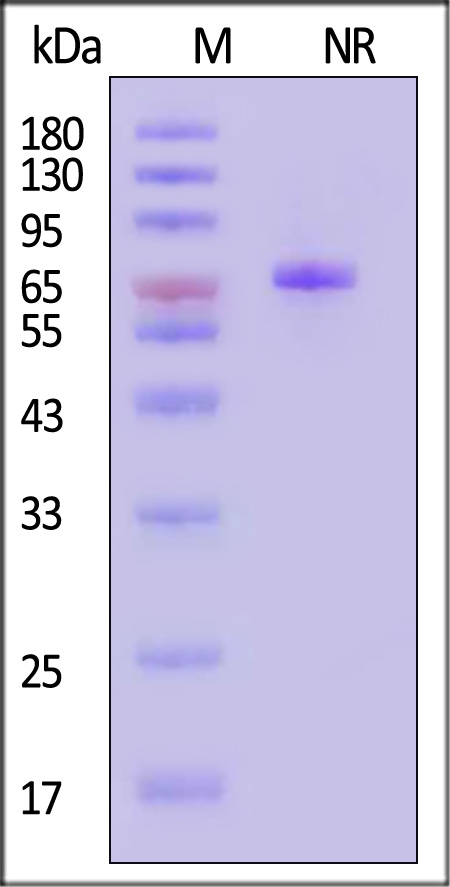
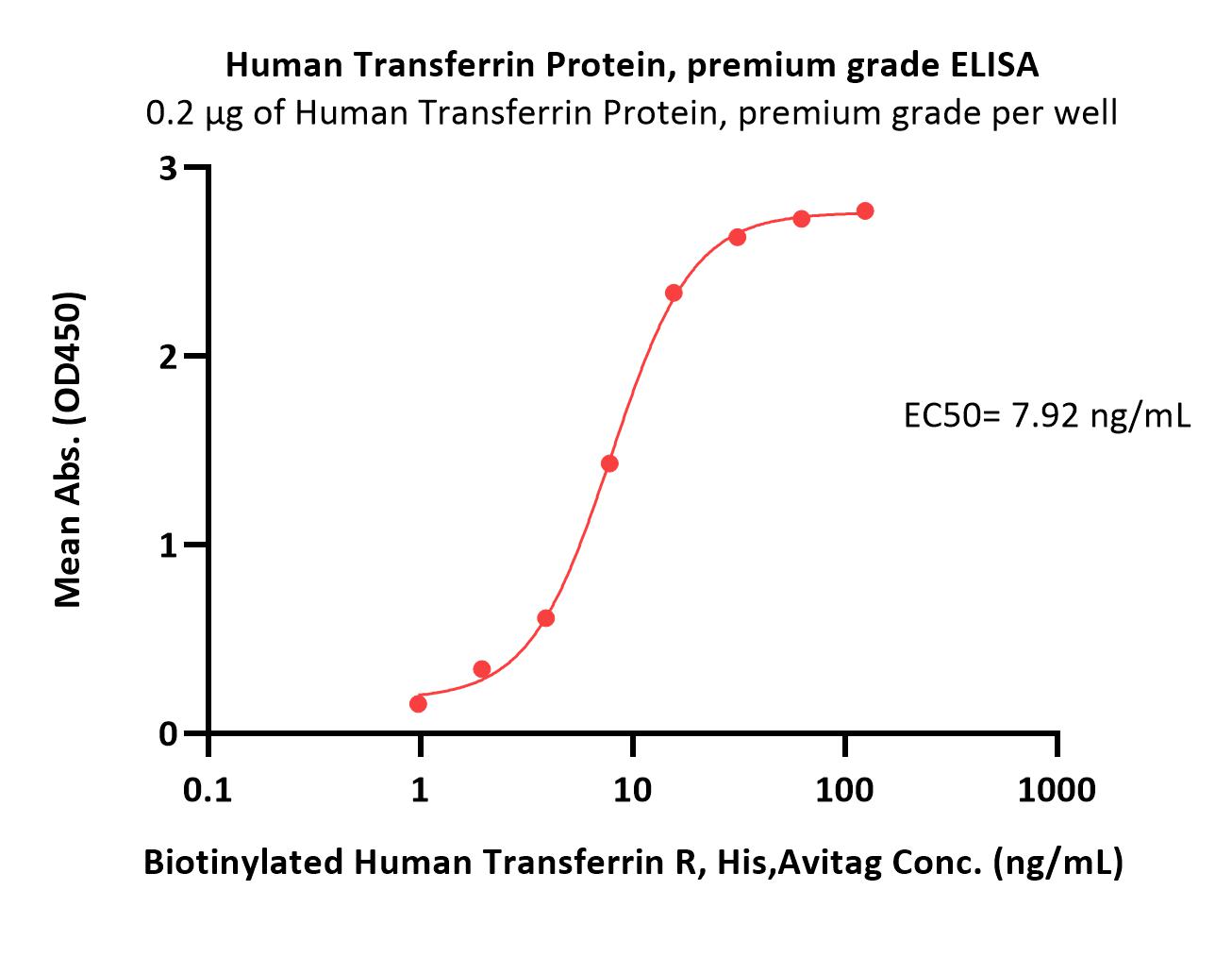
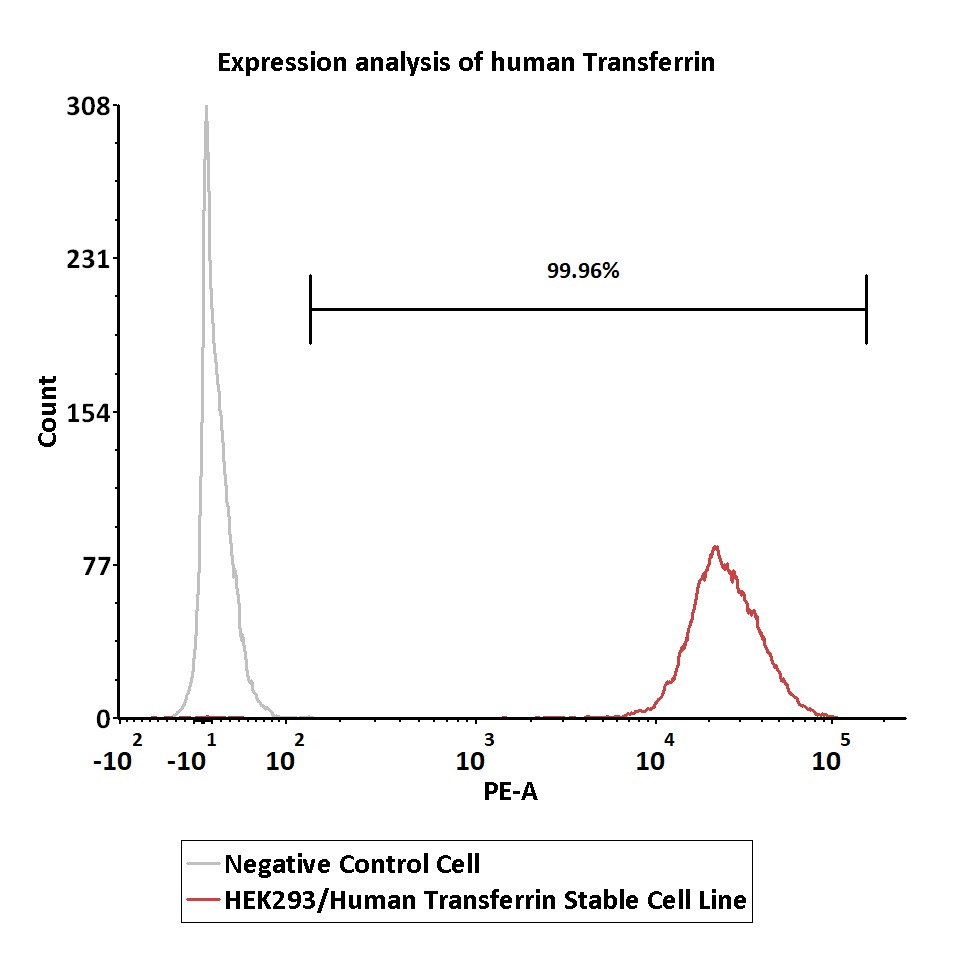
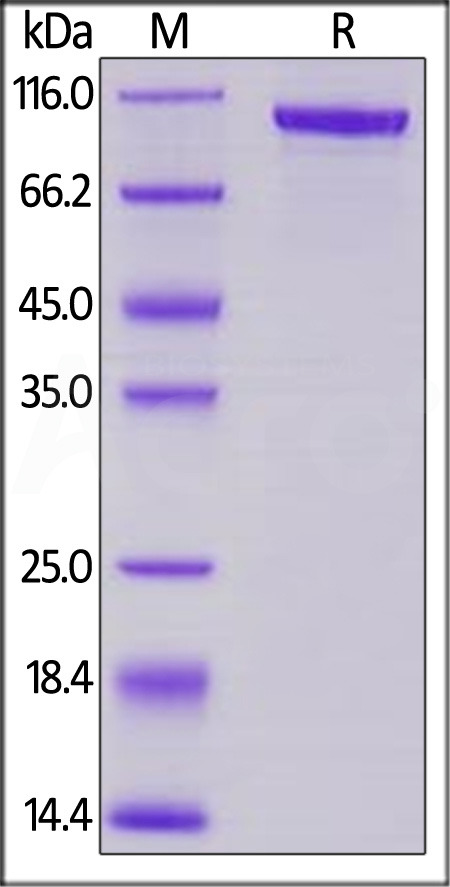
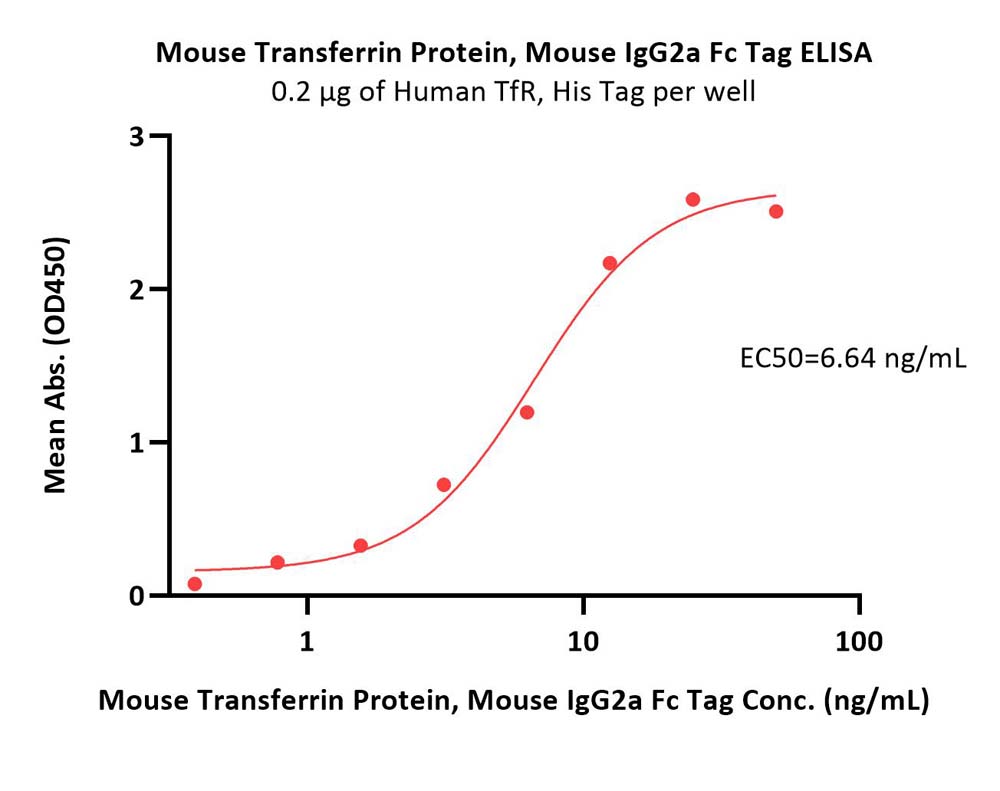
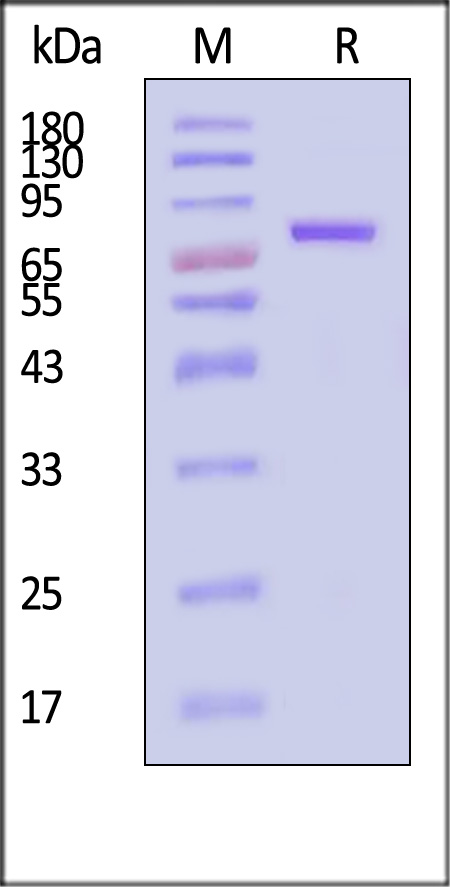
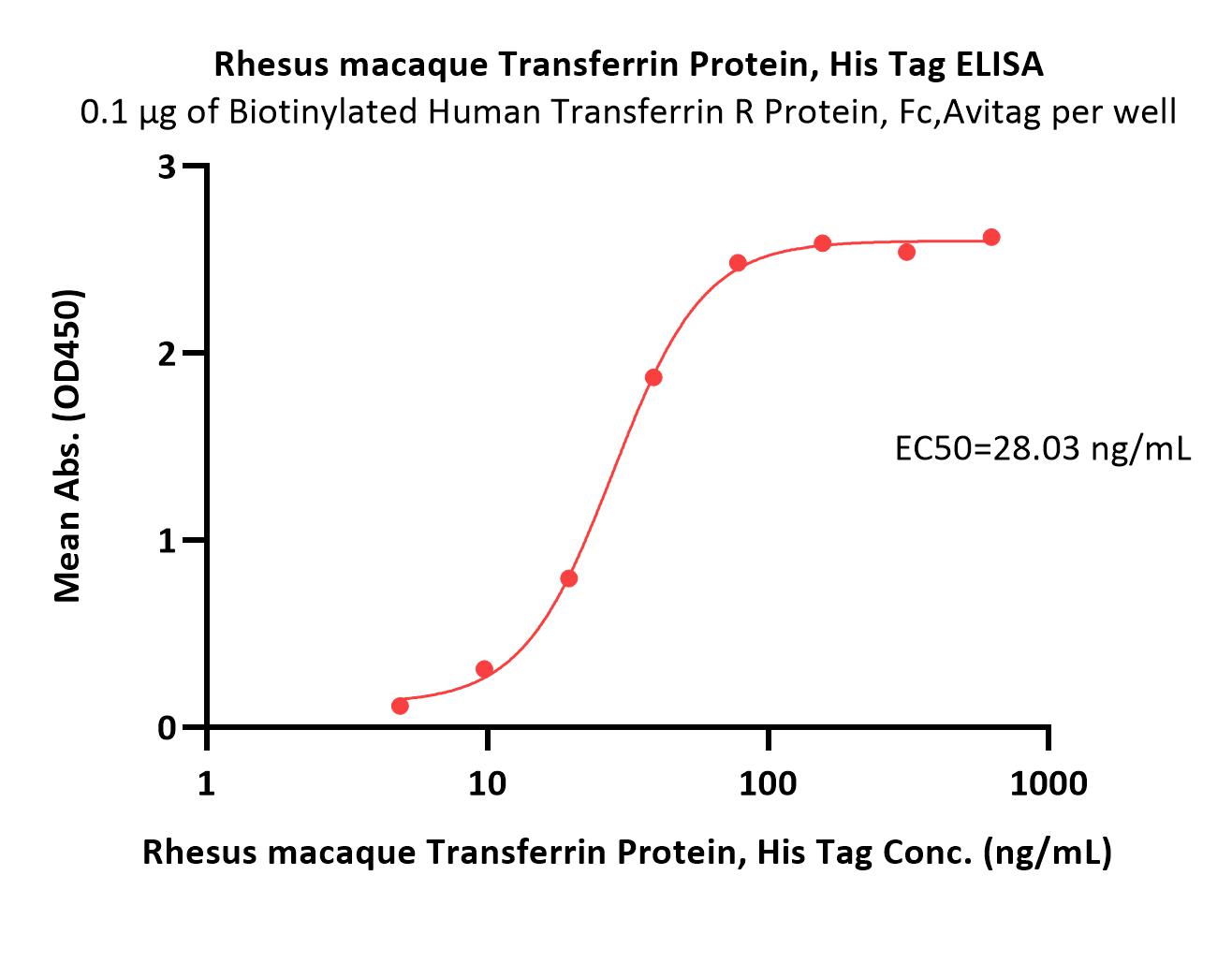
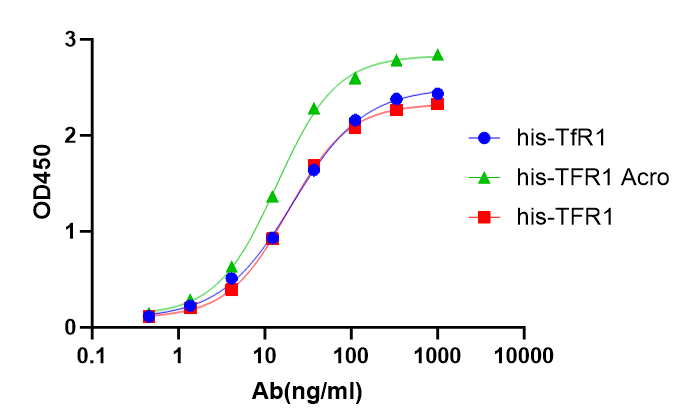
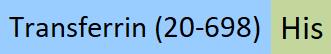Transferrin分子别名
Transferrin,TF,DKFZp781D0156,PRO1557,PRO2086
Transferrin分子背景
Transferrin is also known as Serotransferrin, Beta-1 metal-binding globulin, TF, and is iron-binding blood plasma glycoproteins that control the level of free iron in biological fluids. Although iron bound to transferrin is less than 0.1% (4 mg) of the total body iron, it is the most important iron pool, with the highest rate of turnover (25 mg/24 h). The affinity of transferrin for Fe(III) is extremely high (1023 M−1 at pH 7.4) but decreases progressively with decreasing pH below neutrality.When not bound to iron, it is known as "apo-transferrin”. In humans, transferrin consists of a polypeptide chain containing 679 amino acids. It is a complex composed of alpha helices and beta sheets to form two domains (the first situated in the N-terminus and the second in the C-terminus). The N- and C- terminal sequences are represented by globular lobes and between the two lobes is an iron-binding site. The liver is the main source of manufacturing transferrin, but other sources such as the brain also produce this molecule . Transferrin is also associated with the innate immune system. Transferrin is found in the mucosa and binds iron, thus creating an environment low in free iron that impedes bacteria survival in a process called iron withholding. The level of transferrin decreases in inflammation. The metal binding properties of transferrin have a great influence on the biochemistry of plutonium in humans. Transferrin has a bacteriocidal effect on bacteria, in that it makes Fe3+ unavailable to the bacteria.Carbohydrate deficient transferrin increases in the blood with heavy ethanol consumption and can be monitored via laboratory testing.






















 Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!
Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!































 膜杰作
膜杰作 Star Staining
Star Staining