| 课程回放 |
主题:Virus Contamination Testing and Detection in Biological Products
时间:2020年10月14日 周三 19:30 - 20:30
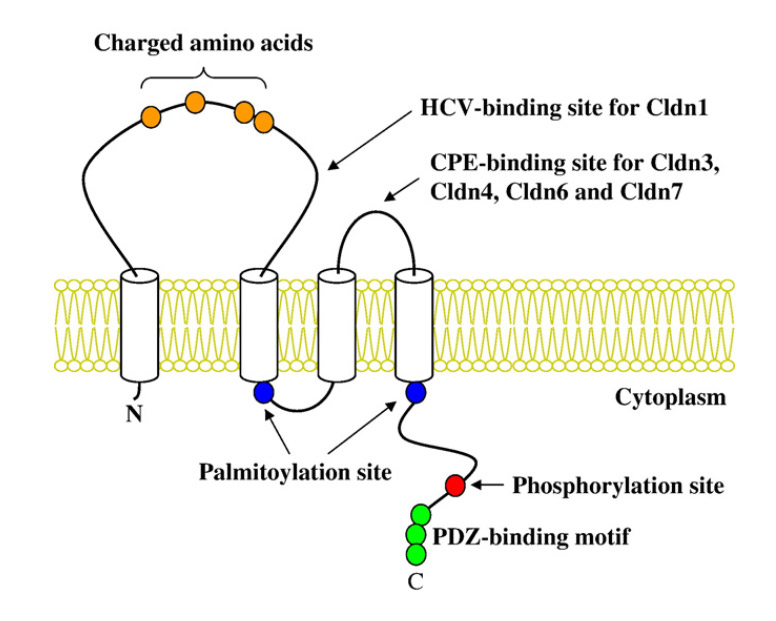
The webinar will present current testing strategies and validated assay methods to ensure biosafety compliance and outline the application of new methods for example next generation sequencing. The testing strategies and methods outlined during this webinar apply in many instances to biosafety testing and characterisation of a range of biologics for example recombinant proteins, monoclonal antibodies, vaccines and gene therapy vectors and cell therapies. SGS Glasgow is a GMP approved testing facility offering a range of validated methods to test a wide range of cell banks and viral vaccine seeds.

Margaret Temple has twenty-five years’ experience of client consultation to discuss and recommend biosafety testing strategies to clients in Asia, North America and Europe. Margaret has worked with clients developing a wide range of biologics: vaccines, cell therapies, gene therapies, recombinant proteins and monoclonal antibodies. Margaret started her career with Q-One Biotech in 1995 and was a co-founder of Vitrology (now SGS Glasgow since acquisition in 2012) with colleagues who remain in the senior management team of SGS Glasgow.
推荐会议更多
ACRO最新 & 热门产品
赢取积分奖励
申请免费参会名额
兑换成功!
免费名额将于3个工作日内发送至您的注册手机和邮箱,
请注意查收~
申请成功!
您已成功申请此次会议资料包,
我们将于2个工作日内将资料包发送至您的邮箱,请注意查收
申请会议资料包
转发分享下面链接后截图上传,即可或得对应会议资料。























































 膜杰作
膜杰作 Star Staining
Star Staining