分子别名(Synonym)
BK14H9.1,BT2.1,BTF1,BTF1BT2.1butyrophilin BTF1,BTN2A1,butyrophilin subfamily 2 member A1,butyrophilin,subfamily 2,member A1,DJ3E1.1,FLJ36567
表达区间及表达系统(Source)
Biotinylated Human BTN2A1, Fc,Avitag (BT1-H52F5) is expressed from human 293 cells (HEK293). It contains AA Gln 29 - Ala 248 (Accession # Q7KYR7-2).
Predicted N-terminus: Gln 29
Request for sequence
蛋白结构(Molecular Characterization)
This protein carries a human IgG1 Fc tag at the C-terminus, followed by an Avi tag (Avitag™).
The protein has a calculated MW of 52.7 kDa. The protein migrates as 65-70 kDa under reducing (R) condition (SDS-PAGE) due to glycosylation.
标记(Biotinylation)
Biotinylation of this product is performed using Avitag™ technology. Briefly, the single lysine residue in the Avitag is enzymatically labeled with biotin.
蛋白标记度(Biotin:Protein Ratio)
Passed as determined by the HABA assay / binding ELISA.
内毒素(Endotoxin)
Less than 1.0 EU per μg by the LAL method.
纯度(Purity)
>95% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in 50 mM Tris, 100 mM Glycine, 25 mM Arginine, 150 mM NaCl, pH7.5. Normally trehalose is added as protectant before lyophilization.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
电泳(SDS-PAGE)

Biotinylated Human BTN2A1, Fc,Avitag on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 95%.
SEC-MALS
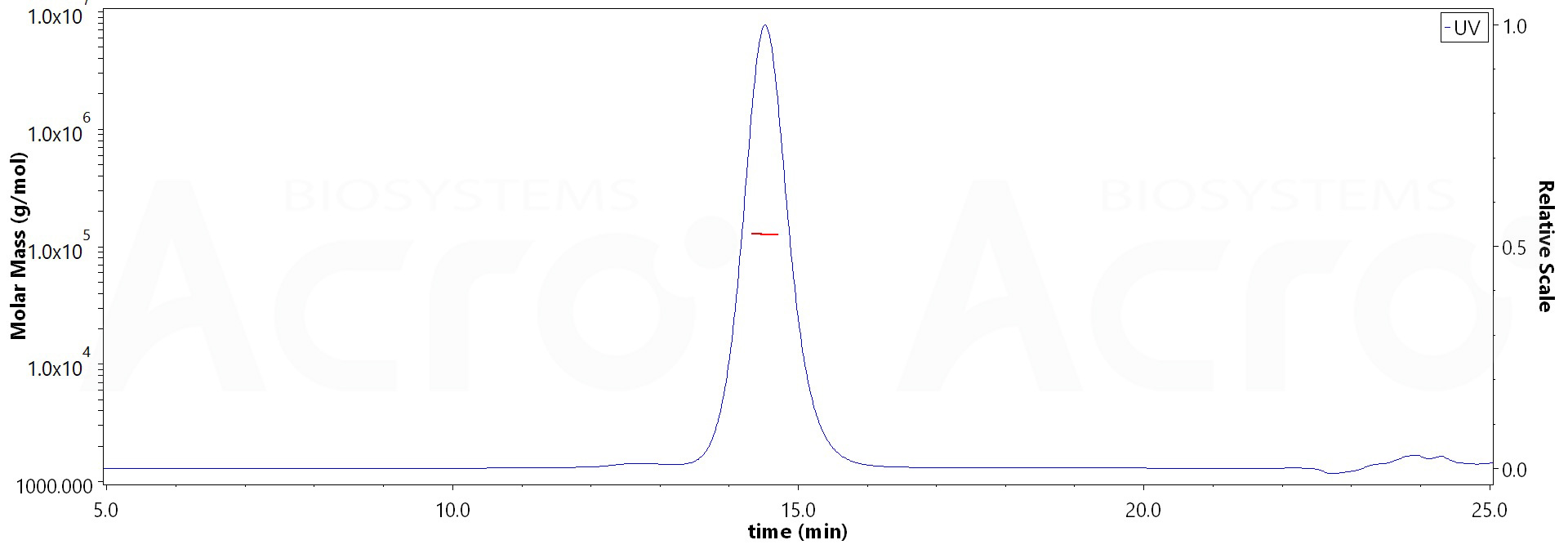
The purity of Biotinylated Human BTN2A1, Fc,Avitag (Cat. No. BT1-H52F5) is more than 90% and the molecular weight of this protein is around 114-139 kDa verified by SEC-MALS.
Report
背景(Background)
Duplication events have led to three paralogues of BTN2A in primates: BTN2A1, BTN2A2, and BTN2A3. In humans, only BTN2A1 has been functionally characterised; it has been detected on epithelial cells and leukocytes, and identified as a novel ligand of dendritic cell-specific ICAM-3 grabbing nonintegrin (DCSIGN), a C-type lectin receptor that acts as an internalization receptor for HIV-1, HCV, and other pathogens. BTN2A2 mRNA has been shown to be expressed in circulating human immune cells.























































 膜杰作
膜杰作 Star Staining
Star Staining
















