抗体来源(Source)
Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) is a chimeric monoclonal antibody recombinantly expressed from CHO, which combines the variable region of a mouse monoclonal antibody with Human constant domain.
克隆号(Clone)
12C6
亚型(Isotype)
Human IgG1 | Human Kappa
偶联(Conjugate)
Unconjugated
抗体类型(Antibody Type)
Recombinant Monoclonal
种属反应性(Reactivity)
Virus
免疫原(Immunogen)
Recombinant HRSV (A) Fusion glycoprotein F0 derived from human 293 cells.
特异性(Specificity)
This product is a specific antibody specifically reacts with Prefusion glycoprotein F0/pre-F protein (RSV).
应用(Application)
| Application | Recommended Usage |
| ELISA | 0.2-625 ng/mL |
纯化(Purification)
Protein A purified / Protein G purified
制剂(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
重构方法(Reconstitution)
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
存储(Storage)
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- -20°C to -70°C for 12 months in lyophilized state;
- -70°C for 3 months under sterile conditions after reconstitution.
质量管理控制体系(QMS)
SEC-MALS
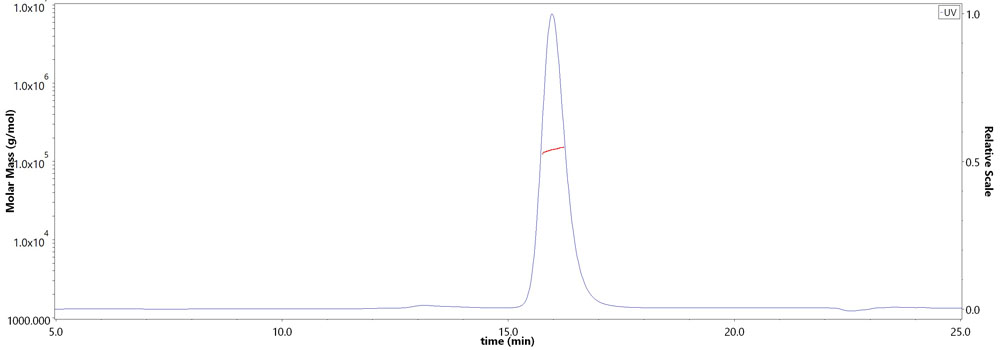
The purity of Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286) is more than 95% and the molecular weight of this protein is around 135-165 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA

Immobilized HRSV (A) Pre-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286) with a linear range of 0.2-2 ng/mL. HRSV (A) Post-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H6) is verified not recoginized by Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286) in low concentration (QC tested).
Protocol

Immobilized HRSV (A) Pre-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286), Anti-Fusion glycoprotein F0 Antibody, Human IgG1 (D25) with a linear range of 0.1-2 ng/mL. HRSV (A) Post-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H6) is verified not recoginized by Monoclonal Anti-RSV-Pre-F0 specific Antibody, Human IgG1 (12C6) (Cat. No. RS0-S286)/Anti-Fusion glycoprotein F0 Antibody, Human IgG1 (D25) in low concentration (Routinely tested).
Protocol
背景(Background)
Human respiratory syncytial virus (HRSV) is the most common etiological agent of acute lower respiratory tract disease in infants and can cause repeated infections throughout life. The RSV fusion glycoprotein (RSV F) is the principal target of RSV neutralizing antibodies in human sera. The RSV F is a type I viral fusion protein synthesized as inactive, single-chain polypeptides that assemble into trimers. RSV F fuses the viral and host cell membranes by irreversible protein refolding from the labile prefusion conformation to the stable post-fusion conformation.























































 膜杰作
膜杰作 Star Staining
Star Staining















