抗体来源(Source)
Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 is a chimeric monoclonal antibody recombinantly expressed from HEK293, which combines the variable region of a mouse monoclonal antibody with Human constant domain.
克隆号(Clone)
hRSV90
亚型(Isotype)
Human IgG1 | Human Kappa
偶联(Conjugate)
Unconjugated
抗体类型(Antibody Type)
Recombinant Monoclonal
特异性(Specificity)
This product is a specific antibody specifically reacts with HRSV F.
应用(Application)
| Application | Recommended Usage |
| ELISA | 0.03-313 ng/mL |
纯度(Purity)
>90% as determined by SDS-PAGE.
>90% as determined by SEC-MALS.
纯化(Purification)
Protein A purified / Protein G purified
制剂(Formulation)
Supplied as 0.2 μm filtered solution in PBS, pH7.4 with trehalose as protectant.
Contact us for customized product form or formulation.
运输(Shipping)
This product is supplied and shipped with dry ice, please inquire the shipping cost.
存储(Storage)
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
- The product MUST be stored at -70°C or lower upon receipt;
- -70°C for 3 months under sterile conditions.
质量管理控制体系(QMS)
电泳(SDS-PAGE)
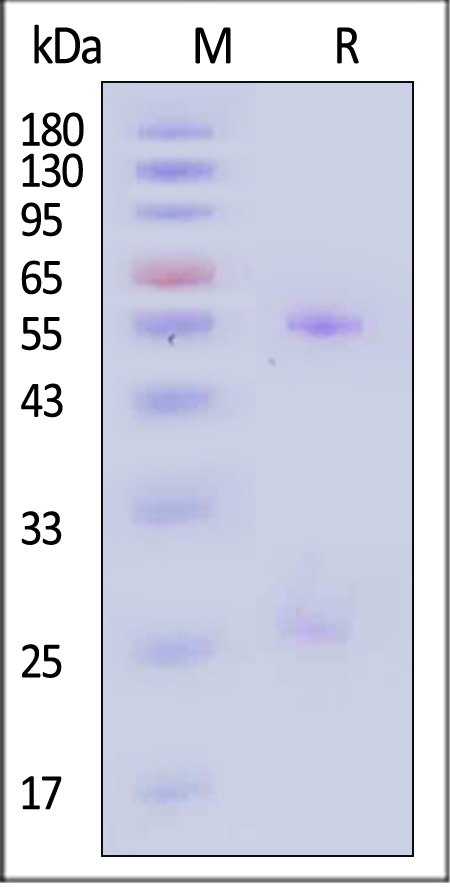
Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 on SDS-PAGE under reducing (R) condition. The gel was stained with Coomassie Blue. The purity of the protein is greater than 90% (With Star Ribbon Pre-stained Protein Marker).
SEC-MALS
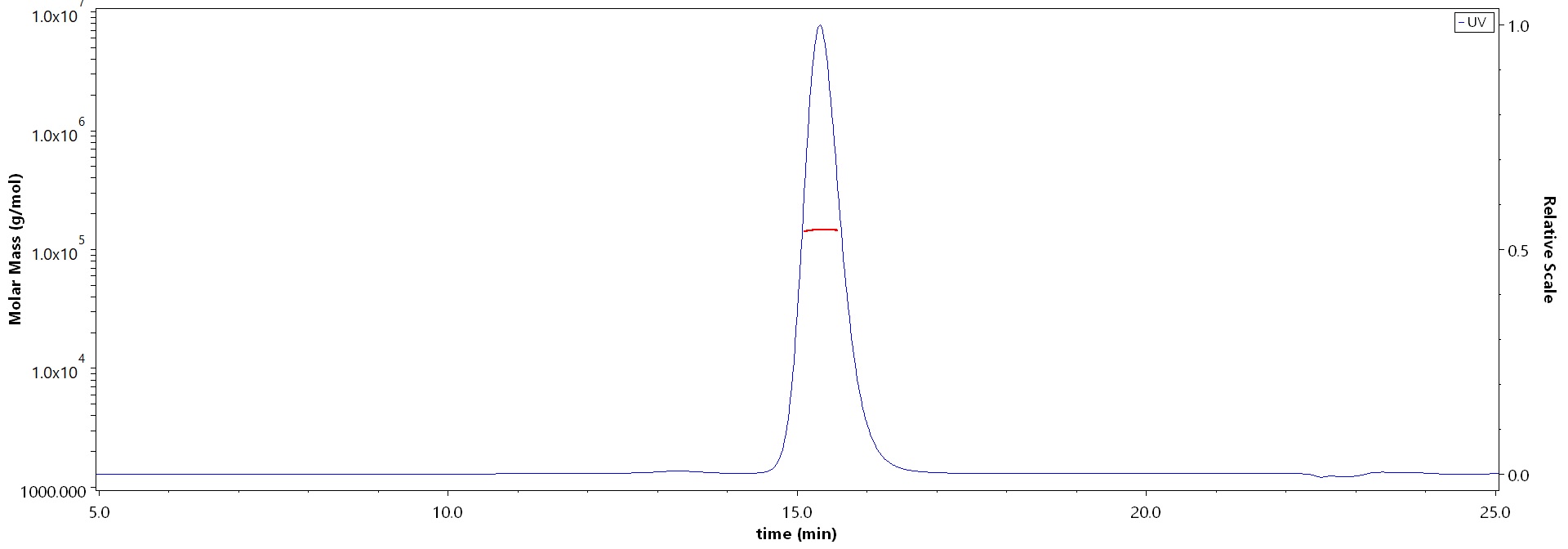
The purity of Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 (Cat. No. HRV-M720) is more than 90% and the molecular weight of this protein is around 135-165 kDa verified by SEC-MALS.
Report
活性(Bioactivity)-ELISA
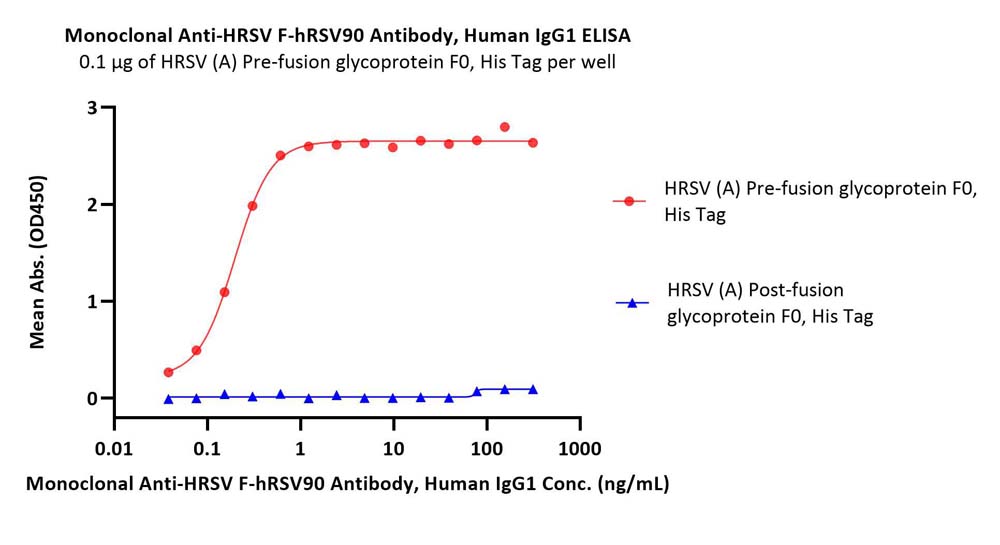
Immobilized HRSV (A) Pre-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 (Cat. No. HRV-M720) with a linear range of 0.03-1 ng/mL. HRSV (A) Post-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H6) is verified not recoginized by Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 (Cat. No. HRV-M720) in low concentration (QC tested).
Protocol
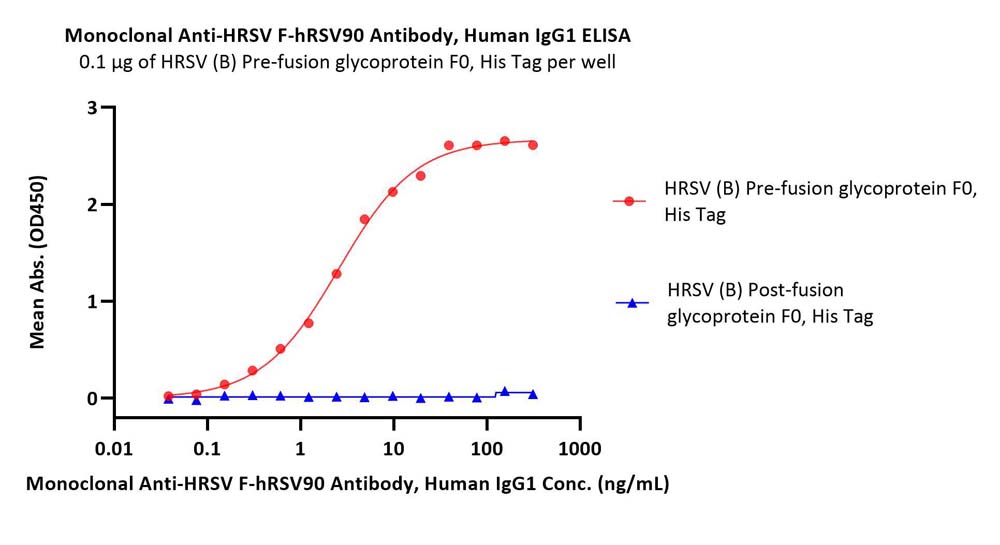
Immobilized HRSV (B) Pre-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H8) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 (Cat. No. HRV-M720) with a linear range of 0.03-1 ng/mL. HRSV (B) Post-fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H9) is verified not recoginized by Monoclonal Anti-HRSV F-hRSV90 Antibody, Human IgG1 (Cat. No. HRV-M720) in low concentration (Routinely tested).
Protocol
背景(Background)
Human respiratory syncytial virus (HRSV) is the most common etiological agent of acute lower respiratory tract disease in infants and can cause repeated infections throughout life. The RSV fusion glycoprotein (RSV F) is the principal target of RSV neutralizing antibodies in human sera. The RSV F is a type I viral fusion protein synthesized as inactive, single-chain polypeptides that assemble into trimers. RSV F fuses the viral and host cell membranes by irreversible protein refolding from the labile prefusion conformation to the stable post-fusion conformation. The antibody hRSV90 heavy chain interacts with residues 163–181 and site Ø, while the light chain interacts with residues 163–181 and site II.























































 膜杰作
膜杰作 Star Staining
Star Staining















